-
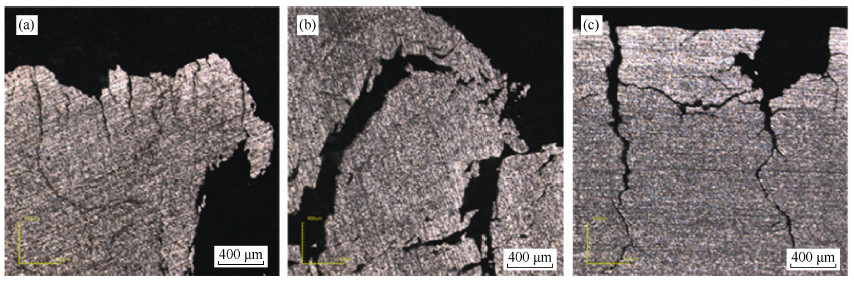
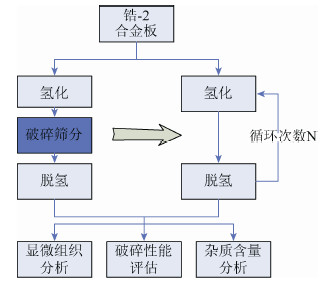
摘要: 在一次氢化-脱氢法的基础上探索锆-2合金粉末制备的循环氢化-脱氢法新工艺。研究了氢化温度和氢化压力对氢化相转变过程的影响,并在此基础上研究了循环次数对氢化脱氢实验过程、氢化锆组织、锆组织及出粉率的影响,结合工艺组织性能研究,探讨了氢化脱氢过程对裂纹扩展的影响机理。研究结果表明:较优的氢化温度为600 ℃,氢化压力为0.3 MPa,循环次数为2次;循环氢化脱氢是两个过程的动态平衡过程,2次循环可以达到一个较优点;循环氢化脱氢的杂质含量控制可以满足要求。Abstract: Based on the single process of hydrogenation-dehydrogenation, a new cyclical hydrogenation-dehydrogenation technology of Zr-2 alloy powder preparation was studied. The effects of hydrogenation temperature and pressure on hydride phase transition were investigated, and the effects of cycle number on hydrogenation-dehydrogenation process, ZrH and Zr structure, and powder productivity were investigated. The influence mechanism of hydrogenation-dehydrogenation process on crack propagation was discussed. In the results, the optimum hydrogenation temperature is 600 ℃, the optimum hydrogenation pressure is 0.3 MPa, and the optimum cycle number is 2 times. Cyclical hydrogenation-dehydrogenation is a dynamic equilibrium process between two hydrogenation-dehydrogenation processes, twice cycle can reach an optimum point. In addition, the impurity controlling can meet the requirement.
-
Keywords:
- hydrogenation /
- dehydrogenation /
- Zr-2 /
- cycle number /
- crack propagation
-
-
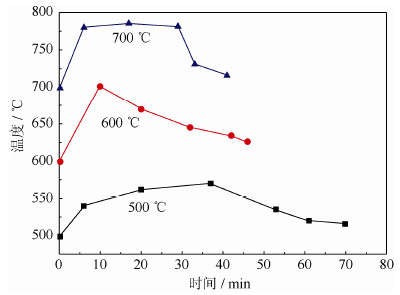
表 1 氢化温度对氢化锆中氢质量分数的影响
Table 1 Influence of hydrogenation temperature on hydrogen content by mass
氢化温度/℃ 500 600 700 氢质量分数/% 1.78 1.72 1.61 表 2 氢化压力对氢质量分数的影响
Table 2 Influence of hydrogenation pressure on hydrogen content by mass
氢化压力/MPa 0.2 0.3 氢质量分数/% 1.71 1.72 表 3 循环次数对氢质量分数影响
Table 3 Influence of cycle number on hydrogen content by mass
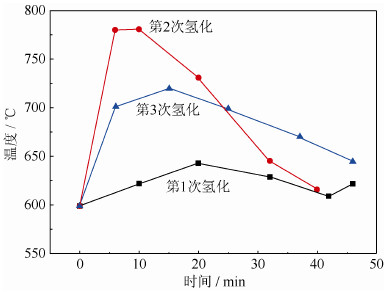
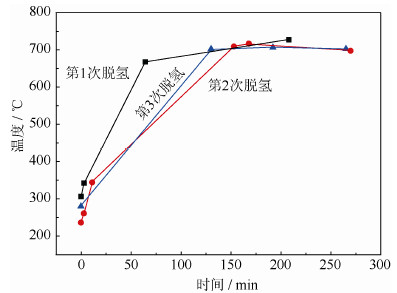
循环次数/次 1 2 3 氢质量分数/% 1.72 1.72 1.69 表 4 循环次数对出粉率影响
Table 4 Influence of cycle number on powder productivity
循环次数/次 出粉率/% 140目 325目 1 10.61 1.32 2 15.44 1.28 3 12.27 1.19 表 5 杂质含量分析
Table 5 Analysis of impurity content
杂质元素 O H N 循环氢化脱氢工艺中杂质含量要求/(μg·g-1) <2500 <25 <120 2次循环样品中杂质含量/(μg·g-1) 2100 22 106 -
[1] 李献军. 锆及锆合金概述. 钛工业进展, 2011, 28(1): 38 DOI: 10.3969/j.issn.1009-9964.2011.01.010 Li X J. Summary of zirconium and zirconium alloys. Titanium Ind Prog, 2011, 28(1): 38 DOI: 10.3969/j.issn.1009-9964.2011.01.010
[2] 熊炳昆. 锆在有色金属材料中的应用. 稀有金属快报, 2005, 24(6): 45 DOI: 10.3969/j.issn.1674-3962.2005.06.014 Xiong B K. The application of zirconium in nonferrous metal materials. Rare Met Lett, 2005, 24(6): 45 DOI: 10.3969/j.issn.1674-3962.2005.06.014
[3] 吴延科, 李庆彬, 徐志高, 等. 金属锆的制备方法. 稀有金属, 2009, 33(4): 462 DOI: 10.3969/j.issn.0258-7076.2009.04.002 Wu Y K, Li Q B, Xu Z G, et al. Review of preparation method of zirconium metal. Chin J Rare Met, 2009, 33(4): 462 DOI: 10.3969/j.issn.0258-7076.2009.04.002
[4] 谢珊珊, 李慧, 张士宪, 等. 金属锆的制备工艺. 热加工工艺, 2017, 46(4): 27 https://www.cnki.com.cn/Article/CJFDTOTAL-SJGY201704007.htm Xie S S, Li H, Zhang S X, et al. Preparation process of metal zirconium. Chin J Rare Met, 2017, 46(4): 27 https://www.cnki.com.cn/Article/CJFDTOTAL-SJGY201704007.htm
[5] 张恒, 沈化森, 车小奎, 等. 氢化-脱氢法制备锆粉工艺研究. 稀有金属, 2011, 35(3): 417 DOI: 10.3969/j.issn.0258-7076.2011.03.018 Zhang H, Shen H S, Che X K, et al. Zirconium powder production through hydrogenation and dehydrogenation process. Chin J Rare Met, 2011, 35(3): 417 DOI: 10.3969/j.issn.0258-7076.2011.03.018
[6] Sun F J, Qu S G, Li G, et al. Comparison of the machinability of titanium alloy forging and powder metallurgy materials. Int J Adv Manuf Technol, 2016, 85(5): 1529 DOI: 10.1007/s00170-015-8045-7
[7] 迟煜頔, 谈萍, 荆鹏, 等. 制备工艺的控制对锆粉性能的影响. 热加工工艺, 2013, 42(12): 48 https://www.cnki.com.cn/Article/CJFDTOTAL-SJGY201312012.htm Chi Y D, Tan P, Jing P, et al. Effects of control of preparation process on properties of zirconium powder. Hot Work Technol, 2013, 42(12): 48 https://www.cnki.com.cn/Article/CJFDTOTAL-SJGY201312012.htm
[8] 单广斌, 魏炳忱, 李金许, 等. 氢对锆基块体非晶合金形变和开裂的影响. 金属学报, 2006, 42(7): 689 DOI: 10.3321/j.issn:0412-1961.2006.07.003 Shan G B, Wei B C, Li J X, et al. Effects of hydrogen on shear bands and cracking in Zr base bulk amorphous alloy. Acta Metall Sinica, 2006, 42(7): 689 DOI: 10.3321/j.issn:0412-1961.2006.07.003
[9] 郭春芳, 董云会. 金属锆制备方法的研究进展. 稀有金属与硬质合金, 2008, 36(2): 63 DOI: 10.3969/j.issn.1004-0536.2008.02.015 Guo C F, Dong Y H. Development of zirconium metal preparation method. Rare Met Cement Carb, 2008, 36(2): 63 DOI: 10.3969/j.issn.1004-0536.2008.02.015
[10] 康建刚, 江垚, 贺跃辉, 等. 氢化-脱氢法制备锆粉及其性能表征. 粉末冶金材料科学与工程, 2015, 20(4): 655 DOI: 10.3969/j.issn.1673-0224.2015.04.025 Kang J G, Jiang Y, He Y H, et al. Fabrication and properties characterization of Zr powder by hydrogenation-dehydrogenation combined method. Mater Sci Eng Powder Metall, 2015, 20(4): 655 DOI: 10.3969/j.issn.1673-0224.2015.04.025
[11] 兰光友, 唐彬, 蒲永兴, 等. 锆合金吸氢及脱氢过程中的组织演变. 核动力工程, 2017, 38(2): 88 https://www.cnki.com.cn/Article/CJFDTOTAL-HDLG201702020.htm Lan G Y, Tang B, Pu Y X, et al. Microstructure evolution in hydrogenation and dehydrogenation process of zircaloy. Nucl Power Eng, 2017, 38(2): 88 https://www.cnki.com.cn/Article/CJFDTOTAL-HDLG201702020.htm
[12] 李圆圆, 朱常桂, 代胜平, 等. 氢化锆合金粉常温氧化与烧结性能研究. 粉末冶金技术, 2012, 30(4): 255 DOI: 10.3969/j.issn.1001-3784.2012.04.003 Li Y Y, Zhu C G, Dai S P, et al. Study of oxidation and sintering behavior of zirconium hydride alloy powder. Powder Metall Technol, 2012, 30(4): 255 DOI: 10.3969/j.issn.1001-3784.2012.04.003
[13] 王涛, 龙剑平, 杨绍利, 等. 氢化钛粉颗粒的粉碎及机理研究. 粉末冶金技术, 2017, 35(3): 208 https://www.cnki.com.cn/Article/CJFDTOTAL-FMYJ201703008.htm Wang T, Long J P, Yang S L, et al. Grinding and mechanism of titanium hydride powder. Powder Metall Technol, 2017, 35(3): 208 https://www.cnki.com.cn/Article/CJFDTOTAL-FMYJ201703008.htm
[14] 张晗亮, 张健, 李增峰, 等. 氢化法制备不饱和氢化钛粉末. 稀有金属, 2006, 30(增刊2): 10 https://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS2006S2002.htm Zhang H L, Zhang J, Li Z F, et al. Preparation of unsaturated titanium hydride powder by hydrogenation process. Chin J Rare Met, 2006, 30(Suppl 2): 10 https://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS2006S2002.htm
[15] 洪艳, 曲涛, 沈化森, 等. 氢化脱氢法制备钛粉工艺研究. 稀有金属, 2007, 31(3): 311 DOI: 10.3969/j.issn.0258-7076.2007.03.007 Hong Y, Qu T, Shen H S, et al. Titanium production through hydrogenation and dehydrogenation process. Chin J Rare Met, 2007, 31(3): 311 DOI: 10.3969/j.issn.0258-7076.2007.03.007
[16] 何薇, 江垚, 杜勇, 等. NaCl包覆/氢化脱氢联合法制备超细钛粉及其性能. 中国有色金属学报, 2012, 22(1): 158 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201201020.htm He W, Jiang Y, Du Y, et al. Fabrication and properties of ultrafine Ti powder by NaCl coated/hydrogenation-dehydrogenation combined method. Chin J Nonferrous Met, 2012, 22(1): 158 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201201020.htm
[17] 熊义富, 陈长安, 张鹏程, 等. 一种铀钼合金粉体的制备方法: 中国专利, CN102240812A. 2011–11–16 Xiong Y F, Chen C A, Zhang P C, et al. Preparation Method for Uranium Molybdenum Alloy Powder: Chinese Patent, CN102240812A. 2011–11–16
-
期刊类型引用(2)
1. 唐培新,吕周晋,车立达,吴战芳. 氧化锌铝陶瓷靶材制备工艺研究. 粉末冶金工业. 2023(04): 22-26 .  百度学术
百度学术
2. 梁浩文,王月,陈小腾,刘正白,白家鸣. 3D打印生物陶瓷人工骨支架的研究进展. 粉末冶金技术. 2022(02): 100-109+117 .  本站查看
本站查看
其他类型引用(0)



 下载:
下载: