-
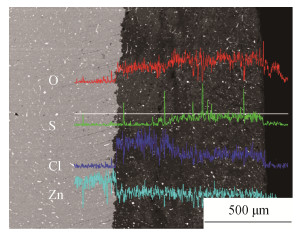
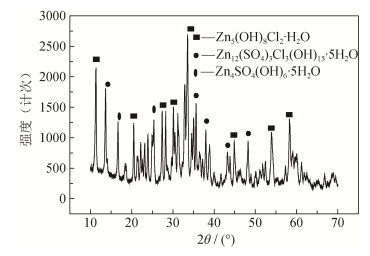
摘要: 通过分析失效锌阳极表面形成的约1 mm厚度的腐蚀产物层发现,腐蚀产物与基体结合牢固、不导电是导致阳极无法发挥保护作用的直接原因。锌阳极表面的腐蚀产物具有明显的分层结构,腐蚀产物形貌以片状产物居多,也有球状产物,片状产物主要为Zn12(SO4)3Cl3(OH)5·5H2O和Zn4SO4(OH)6·5H2O,球状产物主要为Zn5(OH)8Cl2·H2O;锌块中Pb、Cu、Fe含量(质量分数)略微超标,造成锌保护效率下降。当牺牲阳极受到杂散电流干扰,其表面会被快速极化呈钝化状态,以0.1mA·cm-2恒电流极化10 h,锌表面即发生严重钝化,失去其牺牲保护的作用。Abstract: A corrosion product layer in the thickness of about 1 mm was formed on the failed zinc anode surface, and the non-conducting corrosion product was strongly bonded to the substrate, leading to the failure of anode protection. The corrosion products of zinc anode surface showed the obvious layered structure, which was mostly in flake-like and partly in spherical. The sheet products were mainly Zn12(SO4)3Cl3(OH)5·5H2O and Zn4SO4(OH)6·5H2O, and the spherical products were mainly Zn5(OH)8Cl2·H2O; the contents of Pb, Cu, and Fe by mass were slightly exceeded standard in the failed zinc block, resulting in the decrease of zinc protection efficiency. If the sacrificial anode was disturbed by stray current, its surface would be deactivated by rapid polarization; when the sacrificial anode was polarized by the constant current of 0.1 mA·cm-2 for 10 h, the zinc surface would be in polarization passivation state soon, losing the sacrifice protection.
-
Keywords:
- zinc /
- sacrificial anode /
- passivation /
- microstructures /
- failure analysis
-
铝合金是工业中应用最广泛的材料之一,因其具有高强度、高塑性、良好的抗腐蚀性等,被广泛应用在航空航天、汽车、机械制造、船舶及化学工业等领域[1–2]。其中,ZL205A合金是我国自行研制的一种高强高韧铸造合金,其强度超过了7A04、2A50等锻件和部分钢件,并具有机械加工、电镀、抗应力腐蚀等良好的综合性能,适合生产大型受力结构件,并可以用于生产以铸代锻、以铝代钢、整体铸造等构件,同时成本较低,具有较好的经济性,已在飞机、导弹、船舶、汽车等领域内大量使用[3–5]。但是,铝合金硬度较低,耐磨性较差,随着工业经济的飞速发展,对ZL205A合金材料在高速、高载荷和耐磨条件下的使用要求越来越高,为了进一步扩大铝合金的应用范围,就必须在保证其良好基体性能的基础上提高强度和耐磨性。
目前,国内普遍采用表面镀覆、表面合金化、热喷涂、激光熔覆、铸渗法、高能表面改性技术等来增强铝合金的耐磨性[6–10],其中金属基复合材料涂层,尤其是原位颗粒增强金属基复合材料涂层,是金属耐磨材料研究的热点[11]。原位合成颗粒增强金属基复合材料技术克服了外加颗粒增强方式的缺点,具有增强颗粒尺寸小、热力学性能稳定、界面结合强度高等特点[12]。TiC和TiB2是常用的碳化物强化颗粒,具有硬度高、熔点高、热稳定性好等特点[13–14]。
自蔓延高温合成铸渗法是近年来发展起来的一种制备金属基复合材料的方法,将自蔓延高温合成(self-propagation high-temperature synthesis,SHS)技术和铸渗工艺结合,利用铸液的热量维持自蔓延反应,使金属表面原位合成复合强化涂层,从而达到强化金属基体的目的[15–16]。自蔓延高温合成铸渗法使表面合金化、表面复合与真空消失模铸渗过程同时发生,有效解决了铸渗工艺渗剂不易固定、易被金属液冲刷、涂层结合强度不高等问题。
本文将真空消失模铸造技术和自蔓延高温合成技术相结合,在铝基表面原位生成TiC和TiB2复合强化涂层,但是由于铝液浇铸温度较低,铝液经浇铸系统到达SHS粉料处时,温度不足以引燃SHS反应。因此,本文创新地在Ti–C–B4C体系中引入聚四氟乙烯(polytetrafluoroethylene,PTFE),构成Ti–C–B4C–PTFE体系,利用PTFE低温裂解成小分子气体,激发Ti–C–B4C主反应体系的SHS反应,研究ZL205A基Ti–C–B4C–PTFE体系的SHS反应和反应产物,并通过固溶处理,研究分析基体与复合涂层的热稳定。
1. 实验材料及方法
ZL205A合金是高强高韧铸造铝合金的典型代表[3],本文以ZL205A合金为基体,其化学成分如表 1所示。通过自蔓延高温合成反应在铸件表面制备TiC–TiB2的强化涂层,采用AlCu50合金、AlMn20合金、AlTi5合金、AlCd5合金、AlV5合金和纯铝块为熔炼原料,使用钛粉、B4C粉、PTFE粉、石墨为涂层原料,其粉末粒度及纯度列于表 2;自蔓延高温合成反应体系为Ti–C–B4C–PTFE体系,利用真空消失模铸造过程中铝液传递的热量点燃反应,使体系中的Ti和C、B4C原位生成TiC和TiB2增强颗粒,通过铝液渗透作用润湿TiC–TiB2颗粒,最终形成TiC–TiB2颗粒增强铝基表面复合材料。
表 1 基体ZL205A化学成分(质量分数)Table 1. Chemical composition of ZL205A matrix% Cu Ti Cd Mn V Al 4.72 0.20 0.22 0.40 0.10 余量 表 2 涂层原料粉末粒度、纯度和成分质量分数Table 2. Particle size, purity, and mass fraction of coating material powders元素 粒度/ μm 纯度/ % 质量分数/ % Ti ~45 ≥99.7 72.6 B4C ~5 ≥99.0 19.5 PTFE ~10 ≥99.0 4.0 C ~10 ≥99.5 4.1 具体实验过程为:(1)利用加热电阻丝切割泡沫塑料,使之成为具有所需形状的模具;(2)按表 2成分配制涂层原料粉末,以球料比2:1装入球磨罐混粉1 h;(3)配制聚乙烯醇(PVA)水溶液,并与混料粉混合至膏状,然后将混合物涂覆于泡沫塑料模具表面,放入干燥箱干燥,50 ℃下干燥3 h;(4)在干燥后的泡沫塑料模上涂覆耐火涂料,放入干燥箱,在50 ℃下干燥3 h;(5)待完全干燥后,将其埋入砂箱中并用振动台振实;(6)利用中频感应炉熔炼铝合金液,当熔化温度达到780 ℃时,在机械泵抽真空条件下负压浇注;(7)5 min后将砂箱翻砂,空冷得到铸件;(8)按表 3进行固溶处理和时效处理,将铸件在马弗炉中以不同固溶温度保温10 h,在60 ℃温水中冷却,然后在干燥箱中于150 ℃人工时效8 h。
表 3 热处理工艺Table 3. Heat treatment process试样编号 固溶处理 时效处理 固溶温度/ ℃ 保温时间/ h 冷却介质 冷却温度/ ℃ 时效温度/ ℃ 保温时间/ h 冷却介质 0 铸态,不经固溶处理 1 518 10 水 60 150 8 空气 2 528 10 水 60 150 8 空气 3 538 10 水 60 150 8 空气 4 548 10 水 60 150 8 空气 利用DHB-3000型布氏硬度计测试样品的布氏硬度(压头为硬质合金球,载荷2.452 kN,加载时间30 s),并取3点平均值。采用剪切法(YS/T485-2005)表征涂层与基体之间的界面结合强度。通过ML-100型鞘盘回转式磨粒磨损试验机进行SHS涂层与基体的磨损性能测试。使用日本理学Dmax-RC型X射线衍射仪(X-ray diffraction,XRD)鉴定材料的物相组成(铜靶,λ = 0.15406 nm,管电压为40 kV,管电流为150 mA,步长0.02°,扫描速度为2°/min,测量角度范围20°~90°)。利用LEO-1450扫描电镜(scanning electron microscope,SEM)观察试样的显微组织,并用自带能谱仪(energy disperse spectroscope,EDS)定性分析涂层中各物相的化学成分。
2. 结果与讨论
2.1 SHS体系绝热温度计算
当绝热温度(Tad)大于1800 K时,自蔓延反应能够自发进行。图 1所示为Ti–C–B4C–PTFE体系绝热温度(Tad)与PTFE含量(质量分数)关系曲线,可以看出,Ti–C–B4C–PTFE体系为高放热反应体系,当PTFE的加入量在0%~15%(质量分数)时,体系的绝热温度远大于1800 K。当PTFE质量分数为4%时,体系的绝热温度为3003 K,因此自蔓延反应可自发进行。
2.2 X射线衍射分析
图 2所示为SHS反应前后涂层的X射线衍射图谱。可见,SHS反应前涂层中的主要物相是Ti、C和B4C,SHS反应后涂层主要由Al、TiC和TiB2相组成。可以说明,通过真空消失模技术浇注铝液时,引燃Ti–C–B4C–PTFE体系的自蔓延反应,原位生成TiC和TiB2颗粒强化相,可以有效提高铝基表面的耐磨性。在SHS过程中,Ti粉分别与B4C粉和石墨粉发生反应(4Ti+C+B4C→TiC+2TiB2),从而在铝基表面形成TiC–TiB2复合强化涂层。
2.3 硬度分析
图 3所示是热处理前后各组试样的基体和涂层显微硬度值。可以看出,铸态试样的涂层硬度值为HB 284,基体的硬度值为HB 79。经过热处理后,基体的硬度值发生明显变化,而涂层的硬度基本保持不变。当固溶温度为518 ℃时,基体的硬度提高到HB 122,此时涂层的硬度值为HB 285;当固溶温度为528 ℃时,基体的硬度从HB 79提高至HB 137,相对提高了73%;当固溶温度提高至538 ℃时,基体的硬度开始下降。可见,TiC–TiB2的复合涂层的硬度值变化与热处理无关,ZL205A铝基复合涂层试样的最佳固溶温度为528 ℃。
2.4 热稳定性分析
为了进一步分析ZL205A铝基复合涂层试样的热稳定性,研究了不同固溶温度下铝基体与涂层的显微组织变化,图 4所示为热处理前后基体的显微组织形貌。从图 4(a)可以看出,灰黑色区域对应铸态的α-Al基体组织,浅灰色区域为θ(CuAl2)相及T(Al12Mn2Cu)相,主要分布在晶界处,同时基体中存在少量不连续分布的大块物质,主要为含V、Ti、Al及杂质元素的偏析相。经固溶和时效处理后,基体的显微组织明显改善,随着固溶温度的逐步提高,以θ相为主的各种晶界相出现不同程度的溶解,到528 ℃时,基本中已无连续的θ相,如图 4(c)所示。当继续提高固溶温度至538 ℃时,θ相又重新析出,从而影响基体的热稳定性。
图 5为热处理前后涂层的显微组织形貌。结合图 5(a)和表 4可以看出,浅灰色区域主要成分为Ti和C元素(点1),暗灰色区域主要成分为Ti、C和B元素(点2),结合X射线衍射图谱分析结果可知,点1和点2分别为TiC相和TiB2相。对比发现,铸态与热处理后的涂层形貌基体一致,无明显差别。可以看出,TiC–TiB2的复合强化涂层具有良好的热稳定性,其结果与图 3结果一致,随固溶温度的提高,TiC–TiB2复合强化涂层的硬度值基本保持不变。
从图 6(a)涂层低倍扫描电子显微形貌中可以发现,基体与涂层的结合良好。在TiC–TiB2复合涂层中存在铝基体,铝合金作为“粘结相”将涂层粘结在一起,使其呈现较好的冶金结合。在真空消失模铸造过程中,铝合金液一方面引燃Ti–C–B4C–PTFE的自蔓延反应,形成多孔的TiC–TiB2复合涂层;另一方面,铝合金液熔渗进入复合涂层,促进涂层的致密化,并保证涂层与基体的良好结合,其结合强度达到160 MPa。
2.5 耐磨性分析
对2号试样进行磨损实验,并分别施以10、15、20 N的载荷,15 min后测定质量损失,其结果如图 7所示,随着载荷的增加,质量损失增加,但涂层的质量损失量明显低于基体的质量损失量。在10 N载荷作用下,基体的损失量为200.1 mg,涂层的损失量为24.2 mg;当载荷提高至20 N时,基体的损失量为498.6 mg,涂层的损失量为49.7 mg。可以看出,ZL205A铝基体的质量损失量明显,基体的耐磨性较差,采用SHS在基体表面原位生成TiC–TiB2的复合涂层时,可以有效提高基体的耐磨性。
3. 结论
(1)当添加质量分数为4%的PTFE时,Ti–C–B4C–PTFE体系的绝热温度为3003 K,自蔓延反应可自发进行。
(2)在真空消失模铸造ZL205A铝合金过程中,铝合金液引燃Ti–C–B4C–PTFE体系的SHS反应,在基体表面原位形成TiC–TiB2的复合涂层。同时,铝合金液熔渗进入到复合涂层中,促进涂层的致密化,且涂层与基体结合良好。
(3)相比铝基体,TiC–TiB2的复合涂层的硬度值明显较高,在285 HB左右。随着固溶温度的升高,铝基体的硬度呈先升高后降低趋势,以θ相为主的晶界相先溶解后析出,最佳的固溶温度为528 ℃;TiC–TiB2的复合涂层的硬度值基本维持不变,热处理前后的涂层形貌基体一致,说明TiC–TiB2的复合涂层具有良好的热稳定性。
(4)通过在ZL205A铝合金表面原位生成TiC–TiB2的复合涂层,大大提高了铝合金表面的耐磨性。在20 N载荷的作用下,基体的质量损失量为498.6 mg,而涂层的损失量为49.7 mg,损失量相对减少了90%。
-
图 8 失效锌阳极在恒电流极化24 h后样品: (a)、(b) 表面微观形貌; (c) 片状腐蚀产物形貌; (d) 片状腐蚀产物能谱分析; (e) 多孔球状腐蚀产物形貌; (f) 多孔球状腐蚀产物能谱分析
Figure 8. Failure sacrificial anode under a constant current polarization for 24 h in simulated seawater: (a), (b) corrosion products morphology; (c) sheet corrosion products morphology; (d) EDS of sheet corrosion products; (e) porous spherical corrosion products morphology; (f) EDS of porous spherical corrosion products
表 1 失效锌阳极化学成分检测结果(质量分数)
Table 1 Chemical composition results of the failed zinc anode by mass
% 元素 Cu Al Cd Fe Pb Zn 1# 0.1400 0.1100 0.0400 <0.1000 1.3500 余量 2# 0.1400 0.1200 0.0410 <0.1000 1.4200 余量 3# 0.1200 0.1200 0.0460 <0.1000 1.5100 余量 标准含量(质量分数) 0.0020 0.0050 0.0030 0.0014 0.0060 余量 -
[1] Syrett B C, Macdonald D D, Wing S S. Corrosion of copper-nickel alloys in sea water polluted with sulfide and sulfide oxidation products. Corros, 1979, 35(9): 409 DOI: 10.5006/0010-9312-35.9.409
[2] Metikoš-Huković M, Škugor I, Grubač Z, et al. Complexities of corrosion behaviour of copper-nickel alloys under liquid impingement conditions in saline water. Electrochim Acta, 2010, 55(9): 3123 DOI: 10.1016/j.electacta.2010.01.066
[3] Ismail K M, Fathi A M, Badawy W A. Electrochemical behavior of copper-nickel alloys in acidic chloride solutions. Corros Sci, 2006, 48(8): 1912 DOI: 10.1016/j.corsci.2005.07.004
[4] 钱晖, 冯斌, 王加明. 凝汽器铜管硫酸亚铁一次成膜的应用. 湖南电力, 2000, 20(1): 29 https://www.cnki.com.cn/Article/CJFDTOTAL-HNDL200001011.htm Qian H, Feng B, Wang J M. Application of primary ferrous sulfate film formation in copper tube of condenser. Hunan Electr Power, 2000, 20(1): 29 https://www.cnki.com.cn/Article/CJFDTOTAL-HNDL200001011.htm
[5] Wagner P, Little B, Hart K, et al. Environmental fate of sacrificial zinc anodes and influence of a biofilm. Int Biodeterior Biodegrad, 1996, 37(3-4): 151 DOI: 10.1016/S0964-8305(96)00013-3
[6] Dickinson W H, Caccavo Jr F, Lewandowski Z. The ennoblement of stainless steel by manganic oxide biofouling. Corros Sci, 1996, 38(8): 1407 DOI: 10.1016/0010-938X(96)00031-5
[7] 王凤平, 曹丽, 刘照斌, 等. Zn-Al-Cd合金对20#钢牺牲阳极保护行为. 腐蚀科学与防护技术, 2015, 27(5): 431 https://www.cnki.com.cn/Article/CJFDTOTAL-FSFJ201505006.htm Wang F P, Cao L, Liu Z B, et al. Protective performance of Zn-Al-Cd alloy sacrificial anode for 20#steel. Corros Sci Prot Technol, 2015, 27(5): 431 https://www.cnki.com.cn/Article/CJFDTOTAL-FSFJ201505006.htm
[8] 李异, 李永广. 在役海底管线牺牲阳极失效分析. 中国腐蚀与防护学报, 2002, 22(1): 60 DOI: 10.3969/j.issn.1005-4537.2002.01.013 Li Y, Li Y G. The analysis for failure Al sacrificial anode used in sea bed pipelines. J Chin Soc Corros Prot, 2002, 22(1): 60 DOI: 10.3969/j.issn.1005-4537.2002.01.013
[9] Sziráki L, Cziráki A, Geröcs I, et al. A kinetic model of the spontaneous passivation and corrosion of zinc in near neutral Na2SO4 solutions. Electrochim Acta, 1998, 43(1-2): 175 DOI: 10.1016/S0013-4686(97)00223-5
[10] 方景, 陈刚, 司朝霞. 热浸镀锌对铁基粉末冶金零件耐腐蚀性的影响. 粉末冶金技术, 2008, 26(6): 452 https://www.cnki.com.cn/Article/CJFDTOTAL-FMYJ200806013.htm Fang J, Chen G, Si Z X. Effects of hot galvanizing on the corrosion-resistance of iron-based powder metallurgy parts. Powder Metall Technol, 2008, 26(6): 452 https://www.cnki.com.cn/Article/CJFDTOTAL-FMYJ200806013.htm
-
期刊类型引用(2)
1. 卫佳乐,白亚平,刘萌萌,李建平. 自生TiB_2-TiC_x/7075Al基复合材料显微组织与力学性能研究. 西安工业大学学报. 2022(03): 276-284 .  百度学术
百度学术
2. 谢刚,俞小花,彭如振,王有维. 大气等离子喷涂TiB_2涂层微观结构及性能研究. 轻金属. 2020(08): 27-31 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载: