-
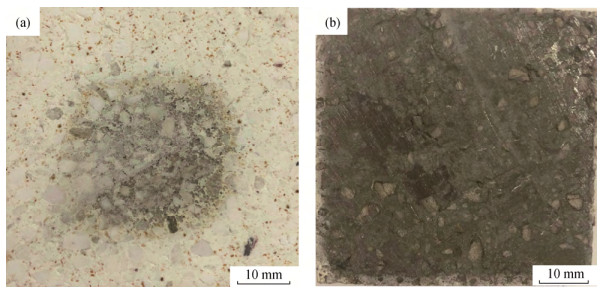
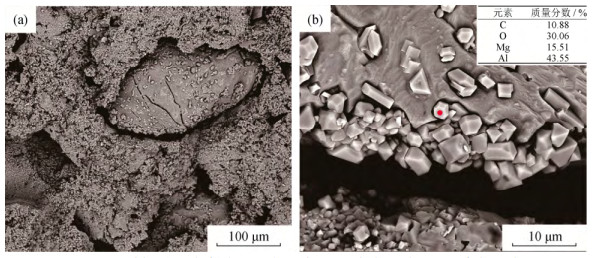
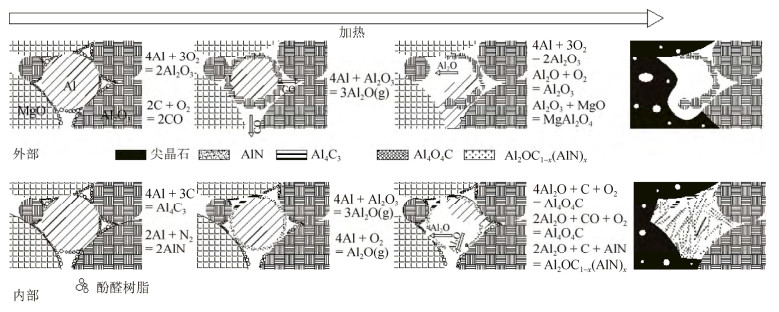
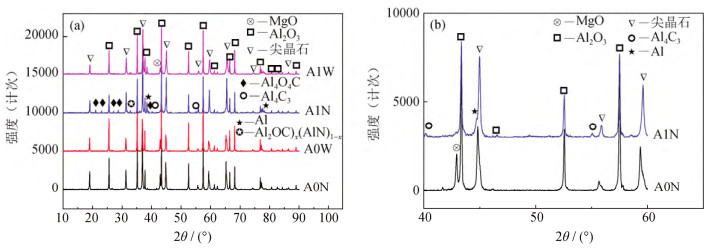
摘要: 以烧结刚玉、α-Al2O3微粉、高纯镁砂、金属铝粉为原料, 酚醛树脂为结合剂, 制备Mg O–Al2O3和Al–MgO–Al2O3系复合材料, 样品成型后经过200℃烘干, 于1500℃氧化气氛烧成。利用X射线衍射仪, 扫描电子显微镜和能谱仪研究了金属铝粉对MgO–Al2O3复合材料抗氧化性的影响。结果表明: 未添加金属铝粉的样品烧后主晶相为α-Al2O3及镁铝尖晶石, 微观结构较为疏松; 引入金属铝粉后, 样品烧后主晶相为α-Al2O3及镁铝尖晶石, 新生相包括Al4O4C、Al4C3、(Al2OC)x(AlN)1-x等, 微观结构较为致密, 样品性能得到改善。添加金属铝粉样品的内外组成呈梯度变化, Al4O4C相主要出现在样品内部, 并有金属铝残留; 金属铝粉引入使样品氧分压从表面到内部依次降低, 金属铝粉氧化后与Mg O原位合成尖晶石, 使结构致密化, 阻隔了氧气的进一步渗入, 样品内部形成的Al2O与C反应得到晶须状Al4O4C。Abstract: MgO–Al2O3 and Al–Mg O–Al2O3 composites were prepared by using the sintered alumina, α-Al2O3 powders, high purity magnesia, and aluminum powders as raw materials, and the phenolic resin as binder. After molding, the samples were dried at 200 ℃ and then sintered at 1500 ℃ in oxidation atmosphere. The effect of aluminum powders on the oxidation resistance of Al2O3–MgO composites were investigated by X-ray diffractometer(XRD), scanning electron microscope(SEM), and energy dispersion spectrometer(EDS). The results show that, the major crystalline phases of the sintered samples without Al addition are α-Al2O3 and MgAl2O4, and the microstructures of the sintered samples are looser. The major crystalline phases of the sintered samples with Al addition are α-Al2O3 and MgAl2O4, and the newly formed phases are Al4O4C, Al4C3, and(Al2OC)x(AlN)1-x; the microstructures of the sintered samples are more densification, and the sintering and properties of samples were improved. The internal and external components of the sintered samples with Al addition show the gradient changes, and the Al4O4C phase mainly appears in the internal region of the sample with residual aluminum. The oxygen partial pressure in the sintered samples decreases gradually from the surface to the interior due to the Al powder addition, the microstructures are densified by spinel, which is in-situ synthesized by oxidizing aluminum powders and MgO, preventing the further infiltration of oxygen. The whisker-like Al4O4C is obtained by the reaction of Al2O formed in the sample and C.
-
Keywords:
- composites /
- aluminum powders /
- MgAl2O4 spinel /
- oxidation resistance
-
-
表 1 原料配比(质量分数)
Table 1 Proportion of raw materials
% 样品编号 烧结刚玉 高纯烧结镁砂(≤1mm) 烧结刚玉(≤44μm) 金属铝粉(≤44μm) α-Al2O3微粉(≤5μm) 3~5mm 1~3mm ≤1mm A0 10 40 15 15 15 — 5 A1 10 40 3 15 15 12 5 表 2 升温制度
Table 2 Heating condition
温度区间/℃ 运行时间/h 烧结速率/(℃·h-1) 室温~580 1 580 580 1(保温) — 580~1500 3 306 1500 3(保温) — 1300~350 随炉冷却 — 表 3 烧成后样品物理性能
Table 3 Physical properties of sintered samples
样品编号 显气孔率/% 密度/(g·cm–3) 常温耐压强度/MPa 线变化率(1500 ℃,2 h)/% A0 25.5 2.76 40 0.92 A1 15.4 2.80 61 1.15 -
[1] 侯金鹏, 娄军峰, 程亮, 等. RH炉耐火材料的使用现状及常见问题分析. 耐火与石灰, 2017, 42(4): 17 https://www.cnki.com.cn/Article/CJFDTOTAL-GWLH201704006.htm Hou J P, Lou J F, Cheng L, et al. The current situation in using RH furnace refractories and analysis for its regular problems. Refract Lime, 2017, 42(4): 17 https://www.cnki.com.cn/Article/CJFDTOTAL-GWLH201704006.htm
[2] 李红霞. RH精炼炉用无铬质耐火材料的研究进展及趋势//中国金属学会第四届中德(欧)冶金技术研讨会论文集. 杜塞尔多夫, 2014: 129 Li H X. Research Progress and Trend of Chromium-Free Refractories for RH Refining Furnace//The Chinese Society for Metals. Fourth Sino-German(European)Seminar on Metallurgical Technology. Dusseldorf, 2014: 129
[3] Belding J H, Letzgus E A. Process for Producing Magnesium Aluminate Spinel: US Patent, US3950504A. 1976‒04‒13.
[4] Magne P, Belser U. Esthetic improvements and in vitro testing of In-Ceram Alumina and Spinell ceramic. Int J Prosthodont, 1997, 10(5): 459. http://europepmc.org/abstract/MED/9495165
[5] Braulio M A L, Rigaud M, Buhr A, et al. Spinel-containing alumina-based refractory castables. Ceram Int, 2011, 37(6): 1705. DOI: 10.1016/j.ceramint.2011.03.049
[6] Ganesh I. A review on magnesium aluminate(Mg Al2O4)spinel: synthesis, processing and applications. Int Mater Rev, 2013, 58(2): 63. DOI: 10.1179/1743280412Y.0000000001
[7] 洪彦若, 孙加林, 王玺堂, 等. 非氧化物复合耐火材料. 北京: 冶金工业出版社, 2003 Hong Y R, Sun J L, Wang X T, et al. Non-Oxide Composite Refractories. Beijing: Metallurgical Industry Press, 2003
[8] Jiang P, Sun J L, Xue W D, et al. New synthetic route to Al4O4C reinforced Al-Al2O3 composite materials. Solid State Sci, 2015, 46: 33. DOI: 10.1016/j.solidstatesciences.2015.05.006
[9] Ma J J, Li Y, Tong S H, et al. Effect of the addition of Al power on the microstructure and phase constitution of Al2O3-Mg O composites sintered at 1700℃under N2atmosphere. Key Eng Mater, 2016, 697: 341. DOI: 10.4028/www.scientific.net/KEM.697.341
[10] Tong S H, Li Y, Yan M W, et al. In situ reaction mechanism of MgAlON in Al-Al2O3-MgO composites at1700℃under flowing N2. Int J Miner Metall Mater, 2017, 24(9): 1061. DOI: 10.1007/s12613-017-1496-0
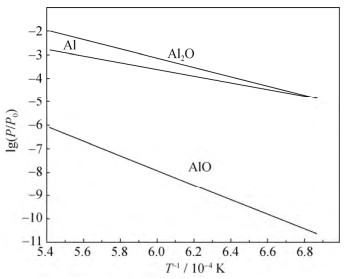
[11] Brewer L, Searcy A W. The gaseous species of the Al-Al2O3 system. J Am Chem Soc, 1951, 73(11): 5308. DOI: 10.1021/ja01155a090
[12] Jiang P, Ma J J, Li Y, et al. Enhanced properties of Mg O-Al2O3 composite materials with Al powder addition under 1300℃creep test and its mechanism analysis. Solid State Sci, 2017, 66: 38. DOI: 10.1016/j.solidstatesciences.2016.12.008
[13] Lihrmann J M, Zambetakis T, Daire M. High-Temperature behavior of the aluminum oxycarbide Al2OC in the system Al2O3-Al4C3 and with additions of aluminum nitride. J Am Ceram Soc, 1989, 72(9): 1704. DOI: 10.1111/j.1151-2916.1989.tb06306.x
[14] Motzfeldt K. Comment on "thermodynamics of the Al-C-O ternary system". J Electrochem Soc, 2007, 154(3): S1. DOI: 10.1149/1.2432077
[15] 陈肇友, 田守信. Al与Si添加剂对Al2O3-C材料抗保护渣侵蚀的影响. 金属学报, 1989, 25(3): B191 https://www.cnki.com.cn/Article/CJFDTOTAL-JSXB198903022.htm Chen Z Y, Tian S X. Effect of Al and Si additives on corrosion resistance of Al2O3-C refractories in mold cover flux. Acta Metall Sinica, 1989, 25(3): B191 https://www.cnki.com.cn/Article/CJFDTOTAL-JSXB198903022.htm
[16] Gokce A S, Gurcan C, Ozgen S, et al. The effect of antioxidants on the oxidation behaviour of magnesia-carbon refractory bricks. Ceram Int, 2008, 34(2): 323. DOI: 10.1016/j.ceramint.2006.10.004
-
期刊类型引用(2)
1. 唐培新,吕周晋,车立达,吴战芳. 氧化锌铝陶瓷靶材制备工艺研究. 粉末冶金工业. 2023(04): 22-26 .  百度学术
百度学术
2. 梁浩文,王月,陈小腾,刘正白,白家鸣. 3D打印生物陶瓷人工骨支架的研究进展. 粉末冶金技术. 2022(02): 100-109+117 .  本站查看
本站查看
其他类型引用(0)



 下载:
下载: