Influence of temperature on the oxidation behaviors of the nickel-based superalloy powders
-
摘要: 通过化学分析、扫描电子显微镜观察、X射线衍射分析及X射线光电子能谱分析等方法, 研究了温度对镍基高温合金粉末氧化行为的影响。结果表明, 室温条件下, 粉末氧含量(质量分数)较低(0.012%), 粉末表面发生部分氧化, 表面存在Ni、Cr、Ti等元素的单质态和以Ni (OH)2、Cr2O3、TiO2为主的氧化物/氢氧化物; 当温度上升至150 ℃, 氧含量增加不明显; 随着温度进一步提高至250 ℃, 粉末氧含量明显增加, 达到0.034%, 粉末表面全部氧化, 表面主要由Ni (OH)2、Cr2O3、TiO2组成。温度对镍基高温合金粉末氧化行为影响显著, 合理控制温度可以获得低氧含量的粉末, 本研究所用镍基高温合金粉末大气条件下最高处理温度为150 ℃。Abstract: Influence of temperature on the oxidation behaviors of the nickel-based superalloy powders was investigated by chemical analysis, scanning electron microscopy (SEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The results indicate that the oxygen content by mass fraction of the nickel-based superalloy powders is 0.012% at room temperature (RT). The oxidation layer partly covers the powder surface in the formation of metallic element (Ni, Cr, Ti) and oxidation state (Ni(OH)2, Cr2O3, TiO2). With the increase of temperature from RT to 150 ℃, the oxygen content seems stable. As the temperature rises to 250 ℃, the oxygen content by mass fraction is 0.034%, showing the obviously increase. The powder surface is completely covered by the oxidation layer at 250 ℃, which is mainly composed of Ni(OH)2, Cr2O3, and TiO2. The elevated temperature significantly affects the oxidation behaviors of the nickel-based superalloy powders, the reasonable control of temperature can obtain the powders with low oxygen content. In this study, the maximum post-process temperature of the nickel-based superalloy powders exposed to the air is 150 ℃.
-
Keywords:
- nickel-based superalloy /
- powders /
- oxidation /
- temperature
-
高温合金是以铁、镍、钴为基体,能在高于600 ℃和一定应力作用下长期工作的一类金属材料。高温合金具有优秀的抗氧化性、抗腐蚀性、抗疲劳性和较高的高温强度、断裂韧性、塑性,在较高温度下表现出良好的组织稳定性和使用可靠性,因此被广泛应用于航空、航天、石油、化工、舰船等领域,主要被用于制造航空发动机、各种工业燃气轮机的关键热端部件[1-5]。
镍基高温合金粉末被广泛应用于增材制造、注射成形、热喷涂等粉末冶金领域,是高温合金的重要研究方向之一[6-8]。作为粉末制件的基础和前提,镍基高温合金粉末直接决定粉末制件的质量和性能。由于比表面积大,镍基高温合金粉末在雾化制备和后续处理过程中容易发生氧化,尤其是在大气暴露和一定温度条件下进行粉末的转移、筛分、除气,极易造成粉末氧含量(质量分数)的增加,从而影响粉末成形过程中粉末颗粒之间的冶金结合,形成粉末原始颗粒边界,最终严重影响合金的力学性能和应用[9-11]。
目前,有关镍基高温合金粉末氧化行为的研究主要集中在对粉末颗粒氧化特征的分析[12-16],缺少温度因素对粉末氧化行为影响的研究。本文采用高纯氩气雾化法制备镍基高温合金粉末,通过扫描电子显微镜(scanning electron microscope, SEM)观察、X射线衍射(X-ray diffraction, XRD)分析及X射线光电子能谱(X-ray photoelectron spectroscopy, XPS)分析等方法,研究了温度对镍基高温合金粉末氧化行为的影响,确定了大气条件下粉末最高处理温度,为粉末后处理工艺提供理论指导。
1. 实验材料及方法
实验用镍基高温合金成分(质量分数)为0.05%C, 15%Cr, 12%Co, 2.5%Al, 2.5%Ti,其余为Ni。首先在高温合金氩气雾化制粉炉中对镍基高温合金进行真空感应熔炼,熔炼真空度≤0.5 Pa,然后采用氩气雾化法制备镍基高温合金粉末,雾化压力为3.0 MPa。
采用标准振动筛筛分获得粒径≤53μm的粉末,取20 g粉末装入刚玉坩埚中,保证粉末在坩埚中分散分布,在箱式电阻炉中进行不同温度的热处理(50、80、100、110、130、150、200、250 ℃),保温2 h。在Leco ONH836氧氮氢分析仪上进行粉末氧含量(质量分数)分析,在此基础上,选取室温(room temperature, RT, 25 ℃)、150 ℃、250 ℃三种温度下的热处理粉末进行后续研究。采用扫描电子显微镜进行粉末表面形貌观察和能谱(energy disperse spectroscope, EDS)分析,利用D/max-RB X射线衍射仪对粉末进行物相分析(Co靶,电压35 kV,电流30 mA,石墨晶体单色器,步进扫描,步长0.02°,积分时间0.4 s),使用PHI Quantera SXM扫描成像X射线光电子能谱对粉末表面氧化行为进行表征(Al靶,X射线束斑200μm,通能55 eV,步长0.1 eV,入射角为45°,分析室真空度优于1.333×10-5 Pa。
2. 结果与讨论
2.1 粉末氧含量分析
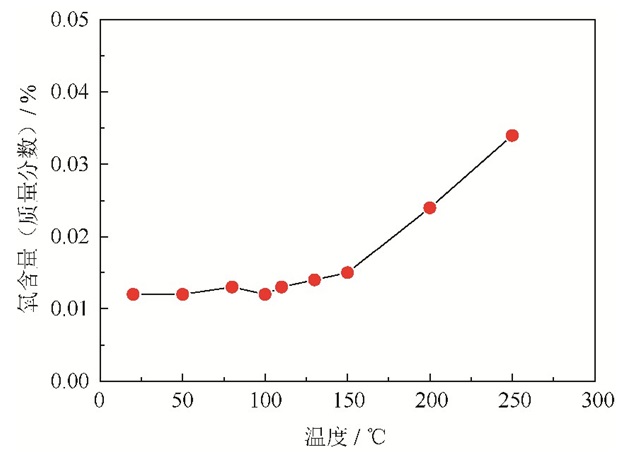
粉末氧含量是影响粉末制件质量和性能的重要因素,对镍基高温合金的后续成形和加工有重要影响。氧元素往往以颗粒表面氧化膜的形式存在,只有氧含量降至0.02%以下,颗粒表面实际上才无氧化膜层。过高的氧含量会形成原始颗粒边界,恶化镍基高温合金的热加工性能,降低力学性能。因此,优质的镍基高温合金粉末必须具有低的氧含量,这也是反映制粉工艺技术水平的重要标志之一[2]。
实验用镍基高温合金粉末在不同热处理温度下氧含量测试结果如图 1所示。由图可知,室温条件下原始粉末的氧含量较低,为0.012%;当加热至50 ℃,粉末氧含量保持不变;当温度达到150 ℃,粉末氧含量为0.015%,与室温相比,粉末氧含量少量增加;随着温度继续提高,粉末氧含量呈明显增加趋势;当加热温度升至200 ℃,粉末氧含量急剧增长至0.024%,甚至在250 ℃时粉末氧含量达到0.034%。因此,对于粒径≤53μm的粉末,确保在大气条件下粉末热处理的温度不超过150 ℃,可防止粉末表面发生严重氧化。
2.2 粉末表面显微形貌分析
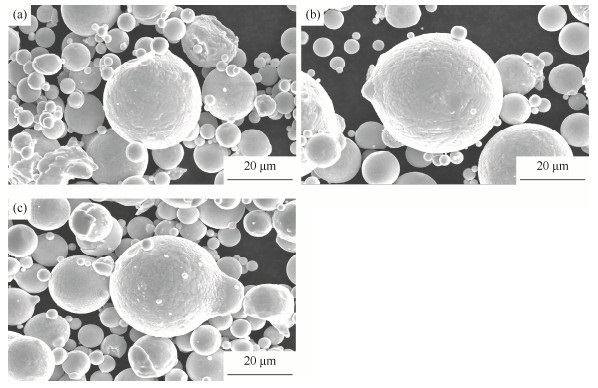
不同热处理温度下镍基高温合金粉末的表面显微形貌如图 2所示。由图可见,镍基高温合金粉末大部分呈规则的球形或近球形,表面较光滑,少数粉末呈棒状等不规则形状。表 1为不同热处理温度下镍基高温合金粉末的能谱分析,其中,室温下原始粉末和150 ℃热处理粉末未探测出氧元素,而250 ℃热处理粉末的氧含量为1.27%。尽管能谱仅能进行定性分析,但仍然可以看出氧含量随温度升高而增加的趋势。室温下原始粉末和150 ℃热处理粉末氧含量较低,无法探测;250 ℃热处理粉末表面氧化较严重,能谱分析有氧元素出现。
表 1 不同热处理温度下镍基高温合金粉末能谱分析Table 1. EDS analysis of the nickel-based superalloy powders at the different thermal treatment temperatures温度/ ℃ 元素质量分数/% C O Al Ti Cr Mn Co Ni 25 5.08 0 2.51 3.10 15.07 0.40 11.79 62.05 150 6.79 0 2.70 2.77 14.79 0.48 11.70 60.77 250 7.62 1.27 2.18 2.25 15.22 0.47 11.45 59.54 2.3 粉末物相分析
图 3为不同热处理温度下镍基高温合金粉末的X射线衍射图谱。由图可知,150 ℃和250 ℃热处理粉末的主要物相组成为基体γ相,与室温原始粉末的物相组成一致,未发现表面氧化物存在。这主要是由于表面氧化物含量较少,低于X射线仪的探测限。
2.4 粉末表面X射线光电子能谱分析
将不同温度处理后的粉末送入真空室进行X射线光电子能谱分析,得到不同热处理温度下镍基高温合金粉末表面的X射线光电子能谱全谱(XPS survey spectrum)。如图 4所示,经不同温度处理后的粉末X射线光电子能谱全谱变化不大,粉末表面成分主要有Ni、Cr、Ti、O、C等元素,未检测到Al、W、Mo、Nb等元素存在。
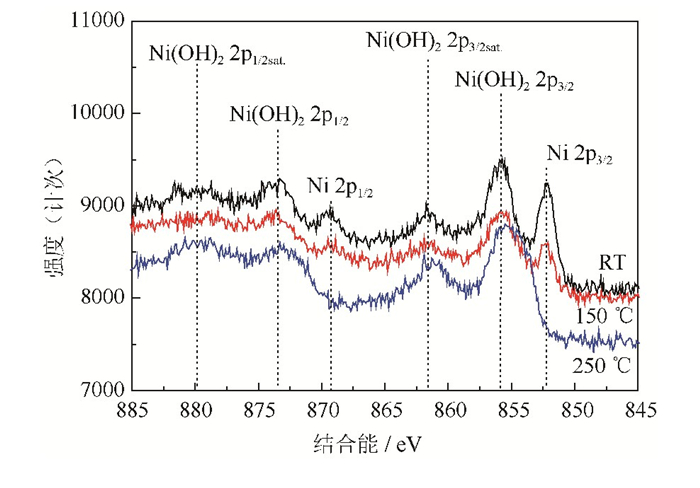
在X射线光电子能谱中,由于电子的自旋–轨道耦合使Ni 2p能级分解为两个能级,即Ni 2p3/2和Ni 2p1/2。图 5是不同热处理温度下粉末表面的Ni 2p窄能量扫描X射线光电子能谱[17-18]。如图所示,室温条件下粉末表面存在明显的Ni 2p3/2和Ni 2p1/2峰,分别在852.4 eV和869.2 eV;同时也存在Ni(OH)22p3/2和Ni(OH)2 2p1/2峰,分别在856 eV和873.5 eV,这与标准Ni、Ni(OH)2谱图及文献报道基本一致[19-20]。由此可知,室温条件下粉末表面Ni元素发生部分氧化,水合反应形成Ni(OH)2,因此粉末表面同时存在单质态Ni和Ni(OH)2;当温度增加至150 ℃,粉末表面的Ni 2p3/2峰明显减弱,说明Ni单质元素减少,Ni元素氧化程度增加;当温度达到250 ℃,粉末表面的Ni 2p3/2和Ni 2p1/2峰消失,而Ni(OH)2 2p3/2和Ni(OH)2 2p1/2峰强度增加,这表明粉末表面Ni元素发生氧化水合,全部形成Ni(OH)2,基本不存在Ni单质。
图 6是不同热处理温度下粉末表面的Ti 2p窄能量扫描X射线光电子能谱。可以看出,室温条件下粉末存在明显的Ti 2p3/2和Ti 2p1/2峰,分别在454.1 eV和460.5 eV;同时也存在TiO2 2p3/2和TiO2 2p1/2峰,分别在458.2 eV和464.0 eV,这与标准Ti及TiO2谱图基本一致。由此可知,室温条件下粉末表面Ti元素发生部分氧化,存在Ti、TiO2两种形式;随着温度增加至150 ℃,Ti 2p3/2峰明显减弱,粉末表面单质态Ti减少,Ti元素氧化程度增加;当温度达到250 ℃,粉末表面的Ti 2p3/2和Ti 2p1/2峰基本消失,TiO2 2p3/2和TiO2 2p1/2峰略有增强,可见粉末表面Ti元素已发生全部氧化,氧化物主要以TiO2为主,粉末表面基本不存在Ti单质。
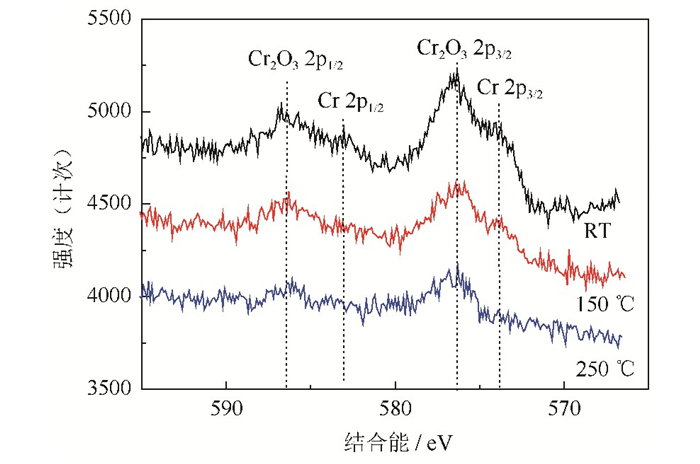
图 7是不同热处理温度下粉末表面的Cr 2p窄能量扫描X射线光电子能谱。由图可知,室温条件下粉末表面存在较强的Cr2O3 2p峰,即处于576.5 eV的Cr2O3 2p3/2峰和586.3 eV的Cr2O3 2p1/2峰;同时还发现了较弱的Cr 2p峰,即处于574.0 eV的Cr 2p3/2峰和583.5 eV的Cr 2p1/2峰,这与标准的Cr及Cr2O3谱图基本一致。因此,室温条件下粉末表面Cr元素发生大部分氧化,主要为Cr2O3,还有少量的Cr单质;加热温度增加至150 ℃,粉末表面Cr 2p3/2峰进一步减弱,Cr元素氧化程度增加;当温度达到250 ℃,粉末表面未发现Cr 2p3/2和Cr 2p1/2峰,仅有Cr2O3 2p峰,可见粉末表面Cr元素已发生全部氧化,氧化物主要以Cr2O3为主,基本不存在Cr单质。
3. 结论
(1) 室温条件下,粉末氧含量(质量分数)较低,为0.012%,粉末表面部分氧化,表面存在Ni、Cr、Ti等元素的单质态和Ni(OH)2、Cr2O3、TiO2为主的氧化物/氢氧化物。
(2) 随着温度的上升(室温上升至150 ℃),氧含量增加不明显,但Ni、Cr、Ti元素单质峰减弱,氧化程度略有增加。
(3) 当温度达250 ℃时,粉末氧含量达到0.034%,粉末表面全部氧化,主要由Ni(OH)2、Cr2O3、TiO2组成。
(4) 温度对镍基高温合金粉末氧化行为影响显著,合理控制温度可以获得低氧的粉末,对本研究所用的镍基高温合金粉末暴露大气条件下最高处理温度为150 ℃。
-
表 1 不同热处理温度下镍基高温合金粉末能谱分析
Table 1 EDS analysis of the nickel-based superalloy powders at the different thermal treatment temperatures
温度/ ℃ 元素质量分数/% C O Al Ti Cr Mn Co Ni 25 5.08 0 2.51 3.10 15.07 0.40 11.79 62.05 150 6.79 0 2.70 2.77 14.79 0.48 11.70 60.77 250 7.62 1.27 2.18 2.25 15.22 0.47 11.45 59.54 -
[1] Reed R C. The Superalloys Fundamentals and Application. London: Cambridge University Press, 2006
[2] 黄乾尧, 李汉康. 高温合金. 北京: 冶金工业出版社, 2000 Huang Q Y, Li H K. Superalloy. Beijing: Metallurgical Industry Press, 2000
[3] Reed R C, Mottura A, Crudden D J. Alloys-by-design: towards optimization of compositions of nickel-based superalloys//Proceedings of The 13th International Symposium of Superalloys. Pennsylvania, 2016: 15
[4] Gessinger G H. Powder Metallurgy of Superalloys. London: Cambridge University Press, 1984
[5] 张健, 楼琅洪. 铸造高温合金研发中的应用基础研究. 金属学报, 2018, 54(11): 1637 DOI: 10.11900/0412.1961.2018.00371 Zhang J, Lou L H. Basic research in development and application of cast superalloy. Acta Metall Sinica, 2018, 54(11): 1637 DOI: 10.11900/0412.1961.2018.00371
[6] Gu Y F, Osada T, Yokokawa T, et al. Development of nickel-cobalt base P/M superalloys for disk applications//Proceedings of The 13th International Symposium of Superalloys. Pennsylvania, 2016: 209
[7] Raisson G. Evolution of PM nickel base superalloy processes and products. Powder Metall, 2008, 51(1): 10 DOI: 10.1179/174329008X286631
[8] 张义文, 刘建涛. 粉末高温合金研究进展. 中国材料进展, 2013, 32(1): 1 https://www.cnki.com.cn/Article/CJFDTOTAL-XJKB201301003.htm Zhang Y W, Liu J T. Development in powder metallurgy superalloy. Mater China, 2013, 32(1): 1 https://www.cnki.com.cn/Article/CJFDTOTAL-XJKB201301003.htm
[9] 傅豪, 王梦雅, 纪箴, 等. 热变形对FGH96高温合金原始颗粒边界的影响. 粉末冶金技术, 2018, 36(3): 201 DOI: 10.19591/j.cnki.cn11-1974/tf.2018.03.007 Fu H, Wang M Y, Ji Z, et al. Effect of thermal deformation on prior particle boundary of FGH96 superalloy. Powder Metall Technol, 2018, 36(3): 201 DOI: 10.19591/j.cnki.cn11-1974/tf.2018.03.007
[10] 马文斌, 刘国权, 胡本芙, 等. 镍基粉末高温合金FGH96中原始粉末颗粒边界的形成机理. 金属学报, 2013, 49(10): 1248 https://www.cnki.com.cn/Article/CJFDTOTAL-JSXB201310013.htm Ma W B, Liu G Q, Hu B F, et al. Formation of previous particle boundaries of nickel base PM superalloy FGH96. Acta Metall Sinica, 2013, 49(10): 1248 https://www.cnki.com.cn/Article/CJFDTOTAL-JSXB201310013.htm
[11] 秦子珺, 刘琛仄, 王子, 等. 镍基粉末高温合金原始颗粒边界形成及组织演化特征. 中国有色金属学报, 2016, 26(1): 50 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201601007.htm Qin Z J, Liu C Z, Wang Z, et al. Formation and microstructure evolution of precipitation on prior particle boundaries in P/M nickel-base superalloys. Chin J Nonferrous Met, 2016, 26(1): 50 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201601007.htm
[12] Gao Z J, Zhang G Q, Li Z, et al. Surface segregation and oxidation behavior of superalloy powders fabricated by argon atomization. Mater Sci Forum, 2013, 747-748: 518 http://www.istic.ac.cn/suoguan/detailed.htm?dbname=xw_qk&wid=0220130700359916
[13] 高正江, 张国庆, 李周, 等. 粉末粒度和氧含量对HIP态FGH96合金组织的影响. 稀有金属, 2012, 36(4): 665 DOI: 10.3969/j.issn.0258-7076.2012.04.026 Gao Z J, Zhang G Q, Li Z, et al. Effect of size distribution and oxygen content of powder on microstructure of HIPed superalloy FGH96. Chin J Rare Met, 2012, 36(4): 665 DOI: 10.3969/j.issn.0258-7076.2012.04.026
[14] 刘娜, 李周, 张国庆, 等. 氩气雾化镍基高温合金粉末的氧化特性研究. 稀有金属, 2011, 35(4): 481 DOI: 10.3969/j.issn.0258-7076.2011.04.002 Liu N, Li Z, Zhang G Q, et al. Oxidation characteristics of nickel-based superalloy powders prepared by argon gas atomization. Chin J Rare Met, 2011, 35(4): 481 DOI: 10.3969/j.issn.0258-7076.2011.04.002
[15] Appa Rao G, Srinivas M, Sarma D S. Effect of oxygen content of powder on microstructure and mechanical properties of hot isostatically pressed superalloy Inconel 718. Mater Sci Eng A, 2006, 435-436: 84 DOI: 10.1016/j.msea.2006.07.053
[16] Xu W Y, Liu Y F, Yuan H, et al. Surface characterization of nickel-base superalloy powder//CMC 2018: Physics and Engineering of Metallic Materials. Xiamen, 2018: 561
[17] Nesbitt H W, Legrand D, Bancroft G M, et al. Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys Chem Miner, 2000, 27: 357 DOI: 10.1007/s002690050265
[18] Watts J F, Wolstenholme J. An Introduction to Surface Analysis by XPS and AES. Chichester: John Wiley & Sons Ltd, 2003
[19] Chasoglou D, Hryha E, Norell M, et al. Characterization of surface oxides on water-atomized steel powder by XPS/AES depth profiling and nano-scale lateral surface analysis. Appl Surf Sci, 2013, 268: 496 DOI: 10.1016/j.apsusc.2012.12.155
[20] Shvab R, Leicht A, Hryha E, et al. Characterization of the virgin and recycled nickel alloy HX powder used for selective laser melting//Proceedings of World PM2016 Congress & Exhibition 2016. Hamburg, 2016: 1692
-
期刊类型引用(1)
1. 尤天伢,纪献兵,郭浩,甘园园. 多孔介质结构特征建模与验证分析. 粉末冶金技术. 2023(02): 154-158+166 .  本站查看
本站查看
其他类型引用(1)



 下载:
下载: