-
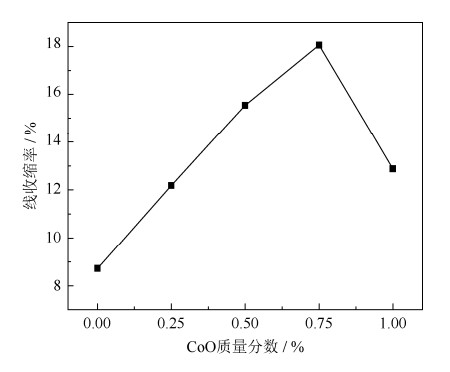
摘要: 通过添加CoO到天然菱镁矿中,采用二步煅烧法制备了镁砂。利用X射线衍射仪和扫描电子显微镜表征了样品的物相构成和显微组织。结果表明,在1600℃烧结保温3 h,未添加CoO试样烧结前后线变化率为8.8%,制备镁砂的体积密度为3.02 g·cm-3,显气孔率为17.8%,MgO晶粒尺寸为3.66 μm;在相同烧结条件下,添加质量分数为0.75% CoO试样烧结前后线变化率为18.1%,镁砂的体积密度为3.28 g·cm-3,显气孔率为9.4%,MgO晶粒尺寸为5.31 μm。经高温烧结后,添加到菱镁矿中的CoO完全固溶到MgO晶格中,改变了MgO晶格常数,导致MgO发生晶格畸变,降低了MgO生长活化能,促进菱镁矿的烧结。Abstract: The magnesia was prepared by using CoO-doped natural magnesite as raw material through two steps calcining process in this paper. Phase components and microstructures of the samples were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. The results show that, the bulk density and apparent porosity of magnesia prepared by magnesite without CoO addition sintered at 1600℃ are 3.02 g·cm-3 and 17.8%, respectively, and the particle size of MgO only is 3.66 μm. In the same sintering conditions, the bulk density and apparent porosity of magnesia prepared by magnesite doped with 0.75% CoO by mass are 3.28 g·cm-3 and 9.4%, respectively, and the particle size of MgO reaches to 5.31 μm. After the high temperature sintering, the CoO-doped natural magnesite changes the lattice constants of MgO and makes the MgO lattice distortion, which consequentially reduces the activation energy of grain growth and promotes the sintering of natural magnesite.
-
Key words:
- magnesite /
- CoO /
- magnesia /
- sintering /
- lattice distortion /
- activation energy
-
表 1 天然菱镁矿石的化学成分(质量分数)
Table 1. Chemical component of natural magnesite
% MgCO3 CaCO3 Fe2O3 SiO2 H2O 95.010 3.020 0.840 1.030 0.013 表 2 添加CoO质量分数与MgO晶粒尺寸的关系
Table 2. Relationship between the CoO content by mass and MgO grain size of magnesite clinker
CoO质量分数/ % MgO晶粒尺寸/ μm 0 3.66 0.25 4.58 0.50 5.19 0.75 5.31 1.00 5.25 表 3 添加不同质量分数CoO的烧结镁砂中MgO晶格常数
Table 3. Lattice constants of MgO in magnesia clinke doped with different CoO contents by mass
CoO质量分数/ % MgO晶格常数/ nm 0 0.420215 0.25 0.420375 0.50 0.420431 0.75 0.420658 1.00 0.420580 -
[1] Yuan Y. Study on the preparation of high purity magnesia by magnesite powder. China Non-met Min Ind Her, 2003(5): 24 doi: 10.3969/j.issn.1007-9386.2003.05.007袁锐. 用菱镁矿粉矿制备高纯镁砂的研究. 中国非金属矿工业导刊, 2003(5): 24 doi: 10.3969/j.issn.1007-9386.2003.05.007 [2] Li H, Su L, Yu J K. Investigation on process flow of high-density magnesia. J Northeastern Univ Nat Sci, 2007, 28(3): 382 https://www.cnki.com.cn/Article/CJFDTOTAL-DBDX200703019.htm李环, 苏莉, 于景坤. 高密度烧结镁砂的研究. 东北大学学报(自然科学版), 2007, 28(3): 382 https://www.cnki.com.cn/Article/CJFDTOTAL-DBDX200703019.htm [3] Zhan D, Huang L, Xiao Z A, et al. Preparation of nanometer magnesium oxide powder by rheology phase-precursor method. Chem Reagent, 2007, 29(3): 141 doi: 10.3969/j.issn.0258-3283.2007.03.005占丹, 黄琳, 肖作安, 等. 流变相-前驱物法制备纳米氧化镁粉体. 化学试剂, 2007, 29(3): 141 doi: 10.3969/j.issn.0258-3283.2007.03.005 [4] Zhu Y X, Zeng R J, Liu X J, et al. Preparation and characterization of MgO nanopowder. J Xiamen Univ Nat Sci, 2001, 40(6): 1256 doi: 10.3321/j.issn:0438-0479.2001.06.015朱亚先, 曾人杰, 刘新锦, 等. MgO纳米粉制备及表征. 厦门大学学报(自然科学版), 2001, 40(6): 1256 doi: 10.3321/j.issn:0438-0479.2001.06.015 [5] Yan W B, Shi A H, Gao F, et al. Synthesis of high purity and nanometer magnesium oxide from light calcined magnesite. J Chin Ceram Soc, 2010, 38(1): 110 https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201001025.htm颜文斌, 石爱华, 高峰, 等. 轻烧菱镁矿制备高纯纳米氧化镁. 硅酸盐学报, 2010, 38(1): 110 https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201001025.htm [6] Cheng C, Ji Z, Jia C C, et al. Effect of MgO addition and sintering temperatures on densification process of Al2O3 ceramics. Powder Metall Technol, 2015, 33(4): 275 doi: 10.3969/j.issn.1001-3784.2015.04.007程诚, 纪箴, 贾成厂, 等. MgO和烧结温度对Al2O3陶瓷致密化过程的影响. 粉末冶金技术, 2015, 33(4): 275 doi: 10.3969/j.issn.1001-3784.2015.04.007 [7] Gui M X. Improvement anti-hydration performance of magnesium sand by adding NiO. Foreign Refract, 2005, 31(3): 35 doi: 10.3969/j.issn.1673-7792.2005.03.010桂明玺. 添加氧化镍对提高镁砂抗水化性所起的效果. 国外耐火材料, 2005, 31(3): 35 doi: 10.3969/j.issn.1673-7792.2005.03.010 [8] Zhen Z J, Li H Y. Rare Earth Functional Materials. 3rd Ed. Beijing: Chemical Industry Press, 2003郑子樵, 李红英. 稀土功能材料. 3版. 北京: 化学工业出版社, 2003 [9] Wang X J. Comprehensive Utilization of Magnesite and the Preparation and Application of Nano-magnesium[Dissertation]. Shanghai: East China Normal University, 2010王小娟. 菱镁矿的综合利用及纳米氧化镁的制备与性能研究[学位论文]. 上海: 华东师范大学, 2010 [10] Zhang W N, Deng N, Liang W J, et al. Effect of CuO on the sintering properties of dolomite. Powder Metall Technol, 2016, 34(4): 277 doi: 10.3969/j.issn.1001-3784.2016.04.008张汪年, 邓宁, 梁伟杰, 等. CuO对白云石烧结性能影响. 粉末冶金技术, 2016, 34(4): 277 doi: 10.3969/j.issn.1001-3784.2016.04.008 [11] Wang C S. Study on MgF2 promoting MgO sintering. Refractories, 1981, 15(6): 1王诚训. MgF2促进MgO烧结的研究. 耐火材料, 1981, 15(6): l [12] Wang Z F, Xu Z W, Zhang B G, et al. Effect of mixture rare earth oxides on microstructure and properties of magnesia refractory. Rare Met Mater Eng, 2007, 36(Suppl 2): 373 https://www.cnki.com.cn/Article/CJFDTOTAL-COSE2007S2109.htm王周福, 徐自伟, 张保国, 等. 混合稀土氧化物对镁质耐火材料结构与性能的影响. 稀有金属材料与工程, 2007, 36(增刊2): 373 https://www.cnki.com.cn/Article/CJFDTOTAL-COSE2007S2109.htm [13] Chaudhuri M, Banerjee G, Kumar A, et al. Secondary phases in natural magnesite sintered with addition of titania, ilmenite and zirconia. J Mater Sci, 1999, 34(23): 5821 doi: 10.1023/A:1004718503991 [14] Lee Y B, Park H C, Oh K D, et al. Sintering and microstructure development in the system MgO–TiO2. J Mater Sci, 1998, 33(17): 4321 doi: 10.1023/A:1004443728590 [15] Martinac V, Labor M, Petric N. Effect of TiO2, SiO2 and Al2O3 on properties of sintered magnesium oxide from sea water. Mater Chem Phys, 1996, 46(1): 23 doi: 10.1016/0254-0584(96)80125-8 [16] Wang X Y, Zhu Y M, Han Y X, et al. Preparation of MgO nano-particles with magnetite. China Powder Sci Technol, 2009, 15(Suppl 1): 49 https://www.cnki.net/KCMS/detail/detail.aspx?dbcode=IPFD&filename=ZJXC200904001017&dbname=IPFD9914王小宇, 朱一民, 韩跃新, 等. 菱镁矿为原料制备纳米氧化镁粉体的工艺研究. 中国粉体技术, 2009, 15(增刊1): 49 https://www.cnki.net/KCMS/detail/detail.aspx?dbcode=IPFD&filename=ZJXC200904001017&dbname=IPFD9914 [17] Liu C M, Zhu X R, Zhou H T. Magnesium Alloy Phase Diagram. 2nd Ed. Changsha: Central South University Press, 2006刘楚明, 朱秀荣, 周海涛. 镁合金相图. 2版. 长沙: 中南大学出版社, 2006 -




 下载:
下载: