Study on spherical silver powders prepared by silver carbonate precursor modified by cladding-thermal decomposition
-
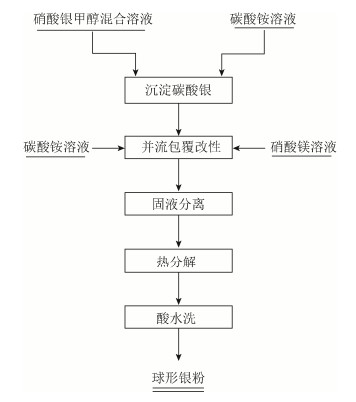
摘要: 以碳酸铵为沉淀剂、硝酸银溶液为原料, 利用化学沉淀法制备得到碳酸银前驱体, 通过并流沉淀法包覆改性碳酸银前驱体, 并经热分解得到单分散的球形银粉。通过X射线衍射分析(X-raydiffraction, XRD)、粒度分布统计(particlesize distribution, PSD)、振实密度测量和扫描电子显微检查(scanning electron microscopy, SEM) 等表征手段研究了热分解银粉的结晶度、纯度、分散性、填充性及微观形貌; 讨论了硝酸银溶液浓度和甲醇添加对碳酸银前驱体颗粒分散性和粒径的影响, 并分析了碳酸镁与碳酸银包覆比例(摩尔比) 对银粉分散性的影响。结果显示, 使用包覆-热分解方法可以制备得到单分散的球形银粉, 该方法具有设备简单、投资少、产品分散性好且粒度分布集中的优点; 当硝酸银溶液浓度为0.2~0.5 mol/L时, 可以得到粒径为0.5~2.5μm的球形银粉; 碳酸银分散性可通过添加甲醇进行调整, 甲醇含量(甲醇在硝酸银溶液中的体积分数) 应控制在5%~10%;当硝酸银溶液浓度为0.5 mol/L、甲醇体积分数为5%时, 碳酸镁与碳酸银摩尔比2:1制备得到的球形银粉分散性最佳。Abstract: The silver carbonate precursor was prepared by chemical precipitation method using ammonium carbonate as precipitation agent and silver nitrate solution as raw material. The cladding silver carbonate precursor was modified by parallel flow precipitation, and the single dispersed spherical silver powders were obtained by the thermal decomposition of modified precursor. The crystallinity, purity, dispersibility, fallibility, and morphology of silver powders were characterized by X-ray diffraction (XRD), particle size distribution (PSD) statistics, tap density test, and scanning electron microscopy (SEM), the influences of silver nitrate solution concentration and methanol adding on the dispersibility and particle size distribution of silver carbonate precursor were discussed, and the effects of cladding ratio (by mole) of magnesium carbonate and silver carbonate on the dispersibility of silver powders were studied. In the results, the single dispersed spherical silver powders can be obtained by cladding-thermal decomposition, which has the advantages of simple equipment, less input, good dispersibility, and the concentrated particle size distribution of products; when the silver nitrate solution concentration is 0.2~0.5 mol/L, the particle size of spherical silver powders is 0.5~2.5 μm; the dispersibility of silver carbonate precursor can be adjusted by adding methanol, the methanol dosage (the volume fraction of methanol in silver nitrate solution) should be controlled as 5%~10%; when the silver nitrate solution concentration is 0.5 mol/L, the methanol dosage by volume is 5%, the optimum dispersibility of silver powders is obtained as the cladding ratio (by mole) of magnesium carbonate and silver carbonate is 2:1.
-
Key words:
- spherical silver powders /
- silver carbonate /
- precursor /
- cladding /
- thermal decomposition
-
表 1 碳酸银前驱体制备条件
Table 1. Preparation conditions of silver carbonate precursor
编号 硝酸银溶液浓度/(mol·L−1) 沉淀剂溶液浓度/(mol·L−1) 温度/℃ 甲醇含量(甲醇在硝酸银溶液中的体积分数)/% 加料方式 Ag2CO3–1 0.2 0.10 40 10 滴加 Ag2CO3–2 0.2 0.10 40 0 滴加 Ag2CO3–3 0.5 0.25 40 5 滴加 Ag2CO3–4 0.5 0.25 40 10 滴加 Ag2CO3–5 0.5 0.25 40 15 滴加 Ag2CO3–6 1.0 0.50 40 20 滴加 表 2 酸洗后热分解球形银粉粒径分布
Table 2. Particle size distribution (PSD) of spherical silver powders prepared by thermal decomposition after acid leaching
粒径尺寸/μm 粒径分布/% 0.463~0.582 0.93 0.582~0.731 8.26 0.731~0.918 35.67 0.918~1.153 41.70 1.153~1.448 12.70 1.448~1.818 0.73 表 3 不同粒径球形银粉物理性能
Table 3. Physical properties of spherical silver powders in different particle sizes
编号 D10/μm D50/μm D90/μm 比表面积/(m2·g-1) 振实密度/(g·cm-3) 灼减/% 平均粒径/μm Ag-A 1.253 1.751 2.346 0.43 5.52 0.29 1.32 Ag-B 0.844 1.010 1.251 0.61 4.44 0.52 0.93 Ag-C 0.677 0.951 1.304 0.88 4.00 0.75 0.65 -
[1] Xie W, Zheng Y Y, Kuang J C, et al. Study on preparation of spherical silver powders with polyvinylpyrrolidone as dispersant. Powder Metall Ind, 2015, 25(1): 23 https://www.cnki.com.cn/Article/CJFDTOTAL-FMYG201501007.htm谢炜, 郑亚亚, 匡加才, 等. 以聚乙烯吡咯烷酮为分散剂制备球形银粉的研究. 粉末冶金工业, 2015, 25(1): 23 https://www.cnki.com.cn/Article/CJFDTOTAL-FMYG201501007.htm [2] Liu S Z, Tan D S, Lv C J. Research on preparation fine silver powder by ascorbic acid reduction. Powder Metall Ind, 2009, 19(2): 5 https://www.cnki.com.cn/Article/CJFDTOTAL-FMYG200902003.htm刘书祯, 谈定生, 吕超君. 抗坏血酸还原制备微细银粉的研究. 粉末冶金工业, 2009, 19(2): 5 https://www.cnki.com.cn/Article/CJFDTOTAL-FMYG200902003.htm [3] Lan X Z, Liu J, Li X Q, et al. Preparation of silver powders by reduction method with hydrazine. Precious Met, 2009, 26(4): 22 https://www.cnki.com.cn/Article/CJFDTOTAL-GJSZ200504006.htm兰尧中, 刘进, 李现强, 等. 水合肼还原法制备超细银粉的研究. 贵金属, 2009, 26(4): 22 https://www.cnki.com.cn/Article/CJFDTOTAL-GJSZ200504006.htm [4] Shuai Y, Wang X B, Zhai H X. Study on process of preparing ultrar-fine spherical silver powder by reduction method with formaldehyde. New Chem Mater, 2011, 39(12): 80 https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201112026.htm帅英, 王献彪, 翟红侠. 甲醛还原制备超细球形银粉的工艺研究. 化工新型材料, 2011, 39(12): 80 https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201112026.htm [5] Li J Y, Wang D X, Sun B S, et al. Characterization of ultrafine nickel powder for MLCC fabricated by coated precursor thermal decomposition method. Powder Metall Technol, 2013, 31(5): 360 doi: 10.3969/j.issn.1001-3784.2013.05.008李军义, 王东新, 孙本双, 等. 包覆分解法制备多层陶瓷电容器用超细Ni粉性能表征. 粉末冶金技术, 2013, 31(5): 360 doi: 10.3969/j.issn.1001-3784.2013.05.008 [6] Hu M Y. Ultra Fine Copper Powder Fabrication and Surface Modification for MLCC Inner Electrode[Dissertation]. Changsha: Central South University, 2008胡敏毅. 多层陶瓷电容器电极用超细铜粉的制备与表面改性研究, 长沙: 中南大学, 2008 [7] Wang J Y, Zhou K G, Jiang Z G. Preparation and size control of spherical cuprous oxide particles by reducing cupric dioxide with glucose. Chin J Inorg Chem, 2011, 27(12): 2405 https://www.cnki.com.cn/Article/CJFDTOTAL-WJHX201112016.htm王岳俊, 周康根, 蒋志刚. 葡萄糖还原氢氧化铜制备球形氧化亚铜及其粒度控制研究. 无机化学学报, 2011, 27(12): 2405 https://www.cnki.com.cn/Article/CJFDTOTAL-WJHX201112016.htm [8] Liu Z H, Liu Z Y, Li Q H, et al. Morphology control of micro-sized spherical silver powder prepared by spray pyrolysis. Chin J Nonferrous Met, 2007, 17(1): 149 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ200701022.htm刘志宏, 刘智勇, 李启厚, 等. 喷雾热分解法制备超细银粉及其形貌控制. 中国有色金属学报, 2007, 17(1): 149 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ200701022.htm [9] Shi W F. Highly Crystalline Silver Powder and Making Method Thereof: China Patent, CN102441675B. 2014-06-01施文锋. 高结晶度银粉的制备方法: 中国专利, CN102441675B. 2014-06-01 [10] Huang K, Guo X Y, Zhang D M. Fundamental theories of particle size and morphology controlling for ultrafine powders in wet chemical precipitation process. Mater Sci Eng Powder Metall, 2005, 10(6): 319 https://www.cnki.com.cn/Article/CJFDTOTAL-FMGC200506000.htm黄凯, 郭学益, 张多默. 超细粉末湿法制备过程中粒子粒度和形貌控制的基础理论. 粉末冶金科学与工程, 2005, 10(6): 319 https://www.cnki.com.cn/Article/CJFDTOTAL-FMGC200506000.htm [11] Zhang C F, Jiang W Y, Zhan L. Preparation of rod precursor of silver powder. Precious Met, 2011, 32(3): 13 https://www.cnki.com.cn/Article/CJFDTOTAL-GJSZ201103002.htm张传福, 蒋伟燕, 湛菁. 形貌控制合成棒状银粉前驱体. 贵金属, 2011, 32(3): 13 https://www.cnki.com.cn/Article/CJFDTOTAL-GJSZ201103002.htm [12] Zheng S L. Powder Surface Modification. Beijing: China Building Material Press, 1995郑水林. 粉体表面改性. 北京: 中国建材工业出版社, 1995 -




 下载:
下载: