Preparation and electrochemical performances of flower-like Co–Ni double hydroxide electrode materials
-
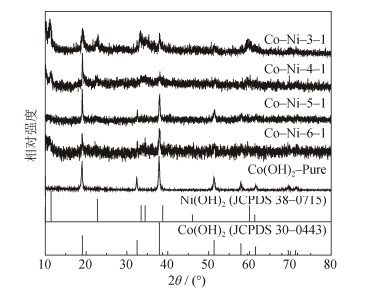
摘要: 采用原位生长法, 以硝酸钴和氨水为原料、硝酸铵为生长剂, 制备生长在泡沫镍上的Co (OH)2电极材料, 并在此基础上对其进行镍添加改性, 旨在得到比电容高、循环性能好的Co–Ni氢氧化物电极材料。通过X射线衍射仪、扫描电子显微镜对Co–Ni氢氧化物电极材料进行物相和微观形貌分析; 通过循环伏安、恒流充放电和交流阻抗等方法对Co–Ni氢氧化物电极材料的电化学性能进行分析和表征。结果表明: 镍添加使材料从原有的Co (OH) 2晶相变为Co (OH) 2和Ni (OH) 2双晶相材料, 使原有的簇状结构转变为更利于离子扩散的花状结构, 进而促进材料电化学性能的提高。当Co/Ni摩尔比为3:1时制得的花状Co–Ni氢氧化物电极材料的电化学性能最好, 在5 m V·s-1扫速下的比电容值为3674.7 F·g-1, 在5 A·g-1电流密度下的比电容值为1450.0 F·g-1, 在20 A·g-1电流密度下循环5000次的比电容保持率为77.1%。Abstract: To obtain the hydroxide electrode materials with excellent capacitance and cycling performance, the cobalt-nickel double hydroxide electrode materials doped by nickel were prepared by in-situ growth method on nickel foams using cobaltous nitrate and ammonium hydroxide as the raw materials and using ammonium nitrate as the promoters.X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used to characterize the crystal phases and microstructures of Co–Ni double hydroxide electrode materials.The tests of cycling voltammetry (CV), chronopotentiometry (CP), and electrochemical impedance spectroscopy (EIS) were conducted to analyze the electrochemical properties of electrode materials.The results show that, the nickel doping results in the crystal phase transform from Co (OH) 2 single-phase to Co (OH) 2 and Ni (OH) 2 bi-phase, and changes the microstructures of electrode materials from cluster-like to flower-like, which is in favor of ion diffusion.Importantly, the flower-like cobalt-nickel double hydroxide electrode material with a Co/Ni mole ratio of 3:1 exhibits the best electrochemical performance, it has a specific capacitance value of 3674.7 F·g -1 at a scan rate of 5 m V·s-1 and that of 1450.0 F·g-1 at a current density of 5 A·g-1, as well as a capacitance retention of 77.1%at 20 A·g-1 after 5000 cycles.
-
Key words:
- cobalt hydroxide /
- in-situ growth /
- modification /
- electrode material /
- supercapacitor
-
图 2 纯Co (OH) 2电极材料、添加不同摩尔分数Ni的Co (OH) 2电极材料和Co–Ni–3–1循环5000次后的扫描电子显微形貌: (a) Co (OH)2–Pure; (b) Co–Ni–6–1; (c) Co–Ni–5–1; (d) Co–Ni–4–1; (e) Co–Ni–3–1; (f) Co–Ni–3–1循环5000次
Figure 2. SEM images of pure Co (OH) 2, Co (OH) 2 electrode materials added by nickel in different mole fractions, and Co–Ni–3–1 after5000 cycles: (a) Co (OH) 2–Pure; (b) Co–Ni–6–1; (c) Co–Ni–5–1; (d) Co–Ni–4–1; (e) Co–Ni–3–1; (f) Co–Ni–3–1 after 5000 cycles
表 1 添加不同摩尔分数镍的Co–Ni氢氧化物电极材料在不同扫描速率下的比电容
Table 1. Specific capacitance values of Co–Ni hydroxide electrode materials added by nickel in different mole fractions at different scanning rates
试样 比电容/(F·g-1) 5mV·s-1 10mV·s-1 20mV·s-1 50mV·s-1 Co(OH)2–Pure 1539.1 1226.8 765.4 471.1 Co–Ni–3–1 3674.7 3346.1 2928.3 2217.4 Co–Ni–4–1 1277.2 1046.0 923.5 611.6 Co–Ni–5–1 2899.8 2455.0 1912.0 1139.1 Co–Ni–6–1 3190.8 2778.6 2321.7 1585.2 表 2 不同电流密度下Co–Ni–3–1的比电容
Table 2. Specific capacitance of Co–Ni–3–1 at various current density
电流密度/(A·g-1) 比电容/(F·g-1) 2 1575 5 1450 10 1340 20 1180 50 825 100 525 200 150 -
[1] Chen S M, Ramachandran R, Mani V, et al. Recent advancements in electrode materials for the high-performance electrochemical supercapacitors: a review. Int J Electrochem Sci, 2014, 9(8): 4072. http://www.researchgate.net/publication/265172767_Recent_Advancements_in_Electrode_Materials_for_the_High-performance_Electrochemical_Supercapacitors_A_Review [2] Tang Z, Tang C H, Gong H. A high energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/carbon nanotube electrodes. Adv Funct Mater, 2012, 22(6): 1272. doi: 10.1002/adfm.201102796 [3] Liu J P, Jiang J, Cheng C W, et al. Co3O4 nanowire@MnO2ultrathin nanosheet core/shell arrays: a new class of high-performance pseudocapacitive materials. Adv Mater, 2011, 23(18): 2076. doi: 10.1002/adma.201100058 [4] Chen K F, Xue D F. Colloidal supercapacitor electrode materials. Mater Res Bull, 2016, 83: 201. doi: 10.1016/j.materresbull.2016.06.013 [5] Wang Y, Guo J, Wang T F, et al. Mesoporous transition metal oxides for supercapacitors. Nanomaterials, 2015, 5(4): 1667. doi: 10.3390/nano5041667 [6] Wan H Z, Miao L, Xu K, et al. Manganese oxide-based electrode behavior as materials for electrochemical supercapacitors. CIESC J, 2013, 64(3): 801 doi: 10.3969/j.issn.0438-1157.2013.03.004万厚钊, 缪灵, 徐葵, 等. MnO2基超级电容器电极材料. 化工学报, 2013, 64(3): 801 doi: 10.3969/j.issn.0438-1157.2013.03.004 [7] Zhu G Y, Chen J, Zhang Z Q, et al. NiO nanowall-assisted growth of thick carbon nanofiber layers on metal wires for fiber supercapacitors. Chem Commun, 2016, 52(13): 2721. doi: 10.1039/C5CC10113A [8] Wang Y, Shi Z Q, Huang Y, et al. Supercapacitor devices based on graphene materials. J Phys Chem C, 2009, 113(30): 13103. doi: 10.1021/jp902214f [9] Wu Z S, Zhou G M, Yin L C, et al. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy, 2012, 1(1): 107. doi: 10.1016/j.nanoen.2011.11.001 [10] Xu B, Zhang H, Cao G P, et al. Carbon materials for supercapacitors. Prog Chem, 2011, 23(2-3): 605 https://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ2011Z1038.htm徐斌, 张浩, 曹高萍, 等. 超级电容器炭电极材料的研究. 化学进展, 2011, 23(2-3): 605 https://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ2011Z1038.htm [11] Hu J Y, Qian F, Song G S, et al. Hierarchical heterostructures of NiCo2O4@XMoO4(X=Ni, Co)as an electrode material for high-performance supercapacitors. Nanoscale Res Lett, 2016, 11(1): 257. doi: 10.1186/s11671-016-1475-9 [12] Li M, Cheng J P, Wang J, et al. The growth of nickel-manganese and cobalt-manganese layered double hydroxides on reduced graphene oxide for supercapacitor. Electrochim Acta, 2016, 206: 108. doi: 10.1016/j.electacta.2016.04.084 [13] Gupta V, Gupta S, Miura N. Electrochemically synthesized large area network of CoxNiyAlz layered triple hydroxides nanosheets: A high performance supercapacitor. J Power Sources, 2009, 189(2): 1292. doi: 10.1016/j.jpowsour.2009.01.026 [14] Cheng J P, Fang J H, Li M, et al. Enhanced electrochemical performance of CoAl-layered double hydroxide nanosheet arrays coated by platinum films. Electrochim Acta, 2013, 114: 68. doi: 10.1016/j.electacta.2013.10.029 [15] Pu J, Tong Y, Wang S B, et al. Nickel-cobalt hydroxide nanosheets arrays on Ni foam for pseudocapacitor applications. J Power Sources, 2014, 250: 250. doi: 10.1016/j.jpowsour.2013.10.108 [16] Jagadale A D, Jamadade V S, Pusawale S N, et al. Effect of scan rate on the morphology of potentiodynamically depositedβ-Co(OH)2 and corresponding supercapacitive performance. Electrochim Acta, 2012, 78: 92. doi: 10.1016/j.electacta.2012.05.137 [17] Yu Z J, Dai Y, Chen W. Electrochemical deposited nanoflakes Co(OH)2 porous films for electrochemical capacitors. J Chin Chem Soc, 2010, 57(3A): 423. doi: 10.1002/jccs.201000063 [18] Yan T, Zhu H Y, Li R Y, et al. Microwave synthesis of nickel/cobalt double hydroxide ultrathin flowerclusters with three-dimensional structures for high-performance supercapacitors. Electrochim Acta, 2013, 111: 71. doi: 10.1016/j.electacta.2013.07.215 [19] Zhang J, Wang X C, Ma J, et al. Preparation of cobalt hydroxide nanosheets on carbon nanotubes/carbon paper conductive substrate for supercapacitor application. Electrochim Acta, 2013, 104: 110. doi: 10.1016/j.electacta.2013.04.052 [20] Guo X L, Liu X Y, Hao X D, et al. Nickel-manganese layered double hydroxide nanosheets supported on nickel foam for high-performance supercapacitor electrode materials. Electrochim Acta, 2016, 194: 179. doi: 10.1016/j.electacta.2016.02.080 [21] Sun Z H, Yuan A B. Electrochemical performance of nickel hydroxide/activated carbon supercapacitors using a modified polyvinyl alcohol based alkaline polymer electrolyte. Chin J Chem Eng, 2009, 17(1): 150. doi: 10.1016/S1004-9541(09)60047-1 -




 下载:
下载: