Influence of sintering temperature on the properties of Li4Ti5O12 anode material for lithium-ion batteries
-
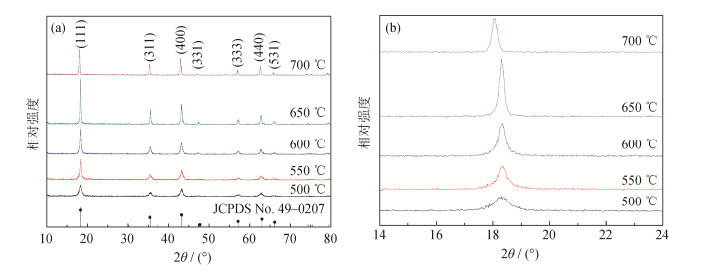
摘要: 采用钛酸四丁酯为钛源、一水合氢氧化锂为锂源,利用水热法制备锂离子电池负极材料Li4Ti5O12(LTO),研究了水热后不同烧结温度对LTO相组成、微观形貌及电化学性能的影响。结果表明:当煅烧温度分别为500、550、600、650、700℃时,烧结LTO均为尖晶石型;500、550、600℃烧结LTO的微观形貌为纳米片状结构,当温度升高到650℃时,LTO出现纳米棒状结构,随着温度继续升高,LTO在700℃时生成较厚的纳米片状结构;当烧结温度为650℃时,LTO的比表面积为94.5907 m2·g-1,气孔体积为0.9663 mL·g-1,此时Li4Ti5O12的放电比容量达到最大值240 mAh·g-1;电流密度100 mA·g-1、循环260次条件下,LTO容量保持率达96.45%,电流密度为1和2 A·g-1、循环1000次条件下,LTO容量保持率达92.97%和77.21%。Abstract: The Li4Ti5O12 (LTO) anode materials for lithium-ion batteries were prepared by hydrothermal method in this paper, using tetrabutyl titanate as the titanium source and lithium hydroxide monohydrate as the lithium source. The influence of sintering temperature on the phase composition, microstructures, and electrochemical properties of LTO was investigated. The results show that the spinel LTO is formed at 500, 550, 600, 650, and 700℃, respectively. The microstructures of LTO are present as the nanosheets sintered at 500, 550, and 600℃, the nanorods sintered at 650℃, and the thicker nonosheets sintered at 700℃. The LTO exhibits the high specific surface area of 94.5907 m2·g-1, the good pore volume of 0.9663 mL·g-1, and the highest initial discharge capacity of 240 mAh·g-1 sintered at 650℃. Additionally, the LTO has an excellent cycling performance with capacity retention ratio of 96.45% after 260 cycles at 100 mA·g-1, and the capacity retention ratio of 92.97% and 77.21% after 1000 cycles at 1 and 2 A·g-1, respectively.
-
Key words:
- lithium ion battery /
- anode material /
- sintering temperature /
- microstructure /
- electrochemical property
-
表 1 不同烧结温度下LTO样品的比表面积和孔体积
Table 1. Specific surface area and pore volume of LTO samples sintered at different temperatures
温度/℃ 比表面积/(m2·g-1) 孔体积/(mL·g-1) 500 37.1633 0.2552 550 71.3419 0.8075 600 85.3326 0.8623 650 94.5907 0.9663 700 45.8839 0.4178 表 2 不同烧结温度下不同电流密度时LTO样品的放电比容量
Table 2. Discharge capacities of LTO samples sintered at different temperatures in different current densities
温度/℃ 放电比容量/(mAh·g-1) 20 mA·g-1 100 mA·g-1 500 m A·g-1 1 A·g-1 2 A·g-1 4 A·g-1 500 142.0 112.9 95.8 86.7 72.7 56.7 550 164.2 141.1 125.1 115.9 103.3 83.4 600 222.4 175.1 152.0 143.6 133.5 119.0 650 240.0 203.8 190.1 184.3 172.6 150.0 700 231.1 177.1 159.6 147.0 133.4 129.3 表 3 不同烧结温度下电解液电阻(RE)和电荷迁移电阻(RCT)数值
Table 3. Electrolyte resistance (RE) and charge transfer resistance (RCT) at different sintering temperatures
温度/℃ RE/Ω RCT/Ω 500 2.71 211.29 550 2.10 127.30 600 4.39 192.01 650 1.80 69.20 700 2.36 93.04 -
[1] Wang Q F, Huang Y, Miao J, et al. Synthesis and electrochemical characterizations of Ce doped SnS2 anode materials for rechargeable lithium ion batteries. Electrochim Acta, 2013, 93(4): 120 http://www.sciencedirect.com/science/article/pii/S0013468613001047 [2] Ji J H, Qin G Q, Qi M F, et al. Preparation and application progress of Li4Ti5O12 anode material. Chin Battery Ind, 2017, 21(5): 45 doi: 10.3969/j.issn.1008-7923.2017.05.012季俊红, 秦国强, 齐满富, 等. Li4Ti5O12负极材料的制备及应用研究进展. 电池工业, 2017, 21(5): 45 doi: 10.3969/j.issn.1008-7923.2017.05.012 [3] Hou L, He X B, Wu M, et al. The influence of carbon content on electrochemical properties of lithium vanadium phosphate. Powder Metall Technol, 2014, 32(6): 403 http://pmt.ustb.edu.cn/article/id/fmyjjs201406001侯磊, 何新波, 吴茂, 等. 碳含量对磷酸钒锂电化学性能的影响. 粉末冶金技术, 2014, 32(6): 403 http://pmt.ustb.edu.cn/article/id/fmyjjs201406001 [4] Yang J, Zhou X Y, Zou Y L, et al. A hierarchical porous carbon material for high power, lithium ion batteries. Electrochim Acta, 2011, 56(24): 8576 doi: 10.1016/j.electacta.2011.07.047 [5] Kan S Y, Yuan M J, Liu F, et al. Mechanism and electrochemical performance of Li4Ti5O12 prepared using Li2TiO3 as raw material. Chin J Nonferrous Met, 2015, 25(3): 697 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201503020.htm阚素荣, 袁敏娟, 刘菲, 等. 以Li2TiO3为原料合成Li4Ti5O12的机理和电化学性能. 中国有色金属学报, 2015, 25(3): 697 https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201503020.htm [6] Yun B N, Du H L, Hwang J Y, et al. Improved electrochemical performance of boron-doped carbon-coated lithium titanate as an anode material for sodium-ion batteries. J Mater Chem A, 2016, 5(6): 1 http://smartsearch.nstl.gov.cn/paper_detail.html?id=2f06764010f34701eaa6c67b7209a2e8 [7] Cheng Q, Tang S, Liu C, et al. Recent advances of Li4Ti5O12 as anode material for high power lithium-ion batteries. J Funct Mater, 2017, 48(12): 12017 https://www.cnki.com.cn/Article/CJFDTOTAL-GNCL201712004.htm程琦, 汤舜, 刘畅, 等. 高倍率钛酸锂负极材料的研究进展. 功能材料, 2017, 48(12): 12017 https://www.cnki.com.cn/Article/CJFDTOTAL-GNCL201712004.htm [8] Fu A A, Zhang Q W, Gao J, et al. Review of anode material Li4Ti5O12 for lithium ion battery. Chin J Power Sources, 2013, 37(12): 2239 doi: 10.3969/j.issn.1002-087X.2013.12.050付安安, 张庆武, 高剑, 等. 锂离子电池负极材料Li4Ti5O12的研究进展. 电源技术, 2013, 37(12): 2239 doi: 10.3969/j.issn.1002-087X.2013.12.050 [9] He Z Q, Xiong L Z, Wu X M, et al. In-situ polymerization method preparation and electrochemical properties of Li4Ti5O12-polyaniline anode material for lithium ion batteries. Chin J Nonferrous Met, 2008, 18(Spec 1): 316 https://cpfd.cnki.com.cn/Article/CPFDTOTAL-ZGYY200804001062.htm何则强, 熊利芝, 吴显明, 等. 原位聚合法制备锂离子电池负极材料Li4Ti5O12-PAn及其电化学性质. 中国有色金属学报, 2008, 18(专辑1): 316 https://cpfd.cnki.com.cn/Article/CPFDTOTAL-ZGYY200804001062.htm [10] Tang B, Li A, Tong Y, et al. Carbon-coated Li4Ti5O12 tablets derived from metal-organic frameworks as anode material for lithium-ion batteries. J Alloys Compd, 2017, 708: 6 doi: 10.1016/j.jallcom.2017.02.279 [11] Sha Y J, Li L, Wei S Y, et al. Appraisal of carbon-coated Li4Ti5O12 acanthospheres from optimized two-step hydrothermal synthesis as a superior anode for sodium-ion batteries. J Alloys Compd, 2017, 705: 164 doi: 10.1016/j.jallcom.2017.02.126 [12] Ming H, Ming J, Li X W. Hierarchical Li4Ti5O12 particles co-modified with C & N towards enhanced performance in lithium-ion battery applications. Electrochim Acta, 2014, 116: 224 doi: 10.1016/j.electacta.2013.11.038 [13] Chang K K, Baben M T, Music D, et al. Estimation of the activation energy for surface diffusion during metastable phase formation. Acta Mater, 2015, 98: 135 doi: 10.1016/j.actamat.2015.07.029 [14] Ni H F. Research on Structural Design and Doping Modification of Li4Ti5O12 Anode Materials [Dissertation]. Beijing: University of Science and Technology Beijing, 2015倪海芳. 钛酸锂负极材料的结构设计及掺杂改性研究[学位论文]. 北京: 北京科技大学, 2015 [15] Chen Y E, Mao H T, Ma J, et al. Biomonitoring chromium Ⅲ or Ⅵ soluble pollution by moss chlorophyll fluorescence. Chemosphere, 2018, 194: 220 doi: 10.1016/j.chemosphere.2017.11.177 [16] Dong S Y, Wang X Y, Shen L F, et al. Trivalent Ti self-doped Li4Ti5O12: A high performance anode material for lithium-ion capacitors. J Electroanal Chem, 2015, 757: 1 doi: 10.1016/j.jelechem.2015.09.002 [17] Bourget L, Corriu R J P, Leclercq D, et al. Non-hydrolytic sol-gel routes to silica. J Non-Cryst Solids, 1998, 242(2-3): 81 doi: 10.1016/S0022-3093(98)00789-3 [18] Aboulaich A, Lorret O, Boury B, et al. Surfactant-free organo-soluble silica-titania and silica nanoparticles. Chem Mater, 2009, 21(13): 2577 doi: 10.1021/cm900291p [19] Kang X H, Jiang H. Effect of different kinds of conductive additives on electrochemical character of Li4Ti5O12. J Beijing Jiaotong Univ, 2010, 34(3): 63 doi: 10.3969/j.issn.1673-0291.2010.03.011康晓红, 江红. 导电剂对锂离子电池负极材料钛酸锂电化学性能的影响. 北京交通大学学报, 2010, 34(3): 63 doi: 10.3969/j.issn.1673-0291.2010.03.011 [20] He Y S, Muhetaer A, Li J M, et al. Ultrathin Li4Ti5O12 nanosheet based hierarchical microspheres for high-rate and long-cycle life Li-ion batteries. Adv Energy Mater, 2017, 7(21): 1700950 doi: 10.1002/aenm.201700950 [21] Yang J W. Development of Li4Ti5O12 Negative Electrode Materials for Hybrid Supercapacitors and Study on Correlative Mechanisms [Dissertation]. Changsha: Central South University, 2005杨建文. Li4Ti5O12基混合超级电容器负极材料的开发及相关机理研究[学位论文]. 长沙: 中南大学, 2005 [22] Pang W K, Peterson V K, Sharma N, et al. Lithium migration in Li4Ti5O12 studied using in situ neutron powder diffraction. Chem Mater, 2014, 26(7): 2318 doi: 10.1021/cm5002779 [23] Pohjalainen E, Rauhala T, Valkeapää M, et al. Effect of Li4Ti5O12 particle size on the performance of lithium ion battery electrodes at high C-rates and low temperatures. J Phys Chem C, 2015, 119(5): 2277 doi: 10.1021/jp509428c -




 下载:
下载: