Synthesis of Ti(C, N) powders by hydrolysis precipitation-carbothemal reduction and nitridation method
-
摘要:
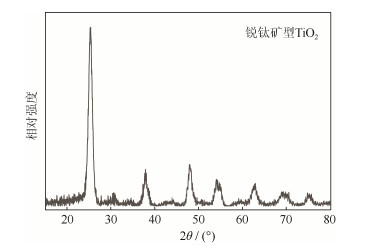
以四氯化钛、炭黑为原料,利用水解沉淀-碳热还原氮化法制备了碳氮化钛粉末。利用差热分析、X射线衍射及扫描电镜等表征手段,研究了合成工艺对粉末物相、组成及形貌等的影响。结果发现:前驱体粉末经350 ℃煅烧2 h后,钛以TiO2的形式存在,TiO2与炭黑形成了混合均匀的团聚体;在碳热还原氮化反应时,钛氧化物向TiCxNyOz转变的温度范围为1200~ 1400 ℃;氮原子促进了钛氧化物向TiCxNyOz的转变,随着反应进一步进行,氧元素逐渐被碳、氮元素置换,形成TiCxNy固溶体;原料经1530 ℃还原4 h后,可合成氧质量分数0.3%、粒度~300 nm、化学式近似为TiC0.547N0.453的碳氮化钛粉末。
Abstract:Ti(C, N) powders were synthesized by the hydrolysis precipitation-carbothemal reduction and nitridation method, using TiCl4 and carbon black as the raw materials. The effects of synthesis technology on the Ti(C, N) powders were characterized by the differential thermal analysis, X-ray diffraction analysis, and scanning electron microscopy. In the results, after baking the precursor powders at 350 ℃ for 2 h, the titanium atoms can only exist in a compound of TiO2, and a uniformly mixed aggregate of TiO2 and carbon black forms. The titanium oxide transforms into a complex compound of TiCxNyOz during the carbothermal reduction and nitridation process at 1200~1400 ℃. The nitrogen atoms accelerate the transformation processes from TiO2 to TiCxNyOz. As the reaction goes on, the oxygen atoms are gradually replaced by carbon and nitrogen atoms to form the solid solution of TiCxNy. The Ti(C, N) powders as the chemical formula of TiC0.547N0.453 are synthesized at 1530 ℃ for 4 h with the oxygen mass fraction of 0.3% and the powder size of ~300 nm.
-
表 1 不同还原时间的碳热还原氮化产物晶面指数与晶胞参数
Table 1. Crystal indices and lattice constants of the carbothermal reduction products at different holding times
晶面指数 还原时间/h 1 2 3 4 2θ/(°) 晶胞参数/nm 2θ/(°) 晶胞参数/nm 2θ/(°) 晶胞参数/nm 2θ/(°) 晶胞参数/nm (220) 61.187 0.42808 61.135 0.42841 61.123 0.42848 61.110 0.42857 (311) 73.267 0.42815 73.203 0.42847 73.190 0.42854 73.163 0.42867 (222) 77.077 0.42828 77.024 0.42853 77.022 0.42853 76.984 0.42871 晶胞参数平均值/nm 0.42817 0.42847 0.42852 0.42865 -
[1] Liu N, Chao S, Yang H D. Cutting performances, mechanical property and microstructure of ultra-fine grade Ti(C, N)-based cermets. Int J Refract Met Hard Mater, 2006, 24(6): 445 doi: 10.1016/j.ijrmhm.2005.09.001 [2] Xu C H, Huang C Z, Ai X. Mechanical property and cutting performance of yttrium-reinforced Al2O3/Ti(C, N) composite ceramic tool material. J Mater Eng Perform, 2001, 10(1): 102 doi: 10.1361/105994901770345411 [3] Kwon W T, Park J S, Kang S. Effect of group Ⅳ elements on the cutting characteristics of Ti(C, N) cermet tools and reliability analysis. J Mater Process Technol, 2005, 166(1): 9 doi: 10.1016/j.jmatprotec.2004.06.009 [4] He X, Ye J W, Liu Y, et al. Studies on the carbothermal preparation of titanium carbonitride powders in the open system. J Funct Mater, 2009, 40(5): 771 doi: 10.3321/j.issn:1001-9731.2009.05.019何旭, 叶金文, 刘颖, 等. 开放体系下碳热还原法制备碳氮化钛粉末的研究. 功能材料, 2009, 40(5): 771 doi: 10.3321/j.issn:1001-9731.2009.05.019 [5] Ke R X, Zhang L, Zhu J F, et al. Preparation and characterization of submicron Ti(C, N) powders produced by carbonthermal reduction-combination reaction. Cement Carb, 2015, 32(2): 71柯荣现, 张立, 朱骥飞, 等. 碳热还原-化合反应制备超细TiCN粉末工艺与特性表征研究. 硬质合金, 2015, 32(2): 71 [6] Yu R H, Wang B Y, Jiang M X, et al. Preparation of titanium carbonitride powders by carbothermal reduction and nitridation method. Refractories, 2006, 40(1): 9 doi: 10.3969/j.issn.1001-1935.2006.01.003于仁红, 王宝玉, 蒋明学, 等. 碳热还原氮化法制备碳氮化钛粉末. 耐火材料, 2006, 40(1): 9 doi: 10.3969/j.issn.1001-1935.2006.01.003 [7] Chen B Q, Ye J W, Liu Y, et al. Preparation of titanium carbonitride powder by carbothermal reduction method. Cement Carb, 2009, 26(2): 98 doi: 10.3969/j.issn.1003-7292.2009.02.007陈帮桥, 叶金文, 刘颖, 等. 碳热还原法制备碳氮化钛粉末. 硬质合金, 2009, 26(2): 98 doi: 10.3969/j.issn.1003-7292.2009.02.007 [8] Yuan Q, Zheng Y, Yu H J. Synthesis of nanocrystalline Ti(C, N) powders by mechanical alloying and influence of alloying elements on the reaction. Int J Refract Met Hard Mater, 2009, 27(1): 121 doi: 10.1016/j.ijrmhm.2008.05.002 [9] Chen X, Xu J F, Xiong W H, et al. Mechanochemical synthesis of Ti(C, N) nanopowder from titanium and melamine. Int J Refract Met Hard Mater, 2015, 50: 152 doi: 10.1016/j.ijrmhm.2015.01.003 [10] Chicardi E, Gotor F J, Alcalá M D, et al. Effects of additives on the synthesis of TiCxN1-x by a solid-gas mechanically induced self-sustaining reaction. Ceram Int, 2018, 44(7): 7605 doi: 10.1016/j.ceramint.2018.01.179 [11] Chen S F, Lu D F, Liu F D, et al. Synthesizing of powder of Ti(C0.12, N0.88) at high temperature. China Ceram, 2000, 36(5): 4陈森凤, 卢迪芬, 刘富德, 等. Ti(C0.12, N0.88)粉末的高温合成. 中国陶瓷, 2000, 36(5): 4 [12] Zgalat-Lozynskyy O, Ragulya A. Densification kinetics and structural evolution during microwave and pressureless sintering of 15 nm titanium nitride powder. Nanoscale Res Lett, 2016, 11(1): 99 doi: 10.1186/s11671-016-1316-x [13] Lin L. Preparation of titanium nitride powders by combustion deoxidization synthesis (Ⅰ) — Theoretical analysis. Dev Appl Mater, 2000, 15(5): 1 doi: 10.3969/j.issn.1003-1545.2000.05.001林立. 燃烧还原化合法制备氮化钛粉末(Ⅰ)—理论分析. 材料开发与应用, 2000, 15(5): 1 doi: 10.3969/j.issn.1003-1545.2000.05.001 [14] Xiang J H, Xiao H N. Synthesis of Ti(C, N) powder by inorganic sol-gol processing. J Inorg Mater, 1998, 13(5): 739 doi: 10.3321/j.issn:1000-324X.1998.05.018向军辉, 肖汉宁. 溶胶-凝胶工艺合成Ti(C, N)超细粉末. 无机材料学报, 1998, 13(5): 739 doi: 10.3321/j.issn:1000-324X.1998.05.018 [15] Shen G Z, Tang K B, An C H, et al. A simple route to prepare nanocrystalline titanium carbonitride. Mater Res Bull, 2002, 37(6): 1207 doi: 10.1016/S0025-5408(02)00736-5 [16] Chen R C. Study on the hydrolysis process of TiCl4. Hydrometall China, 1999(3): 1 https://www.cnki.com.cn/Article/CJFDTOTAL-SFYJ199903000.htm陈瑞澄. 四氯化钛水解过程的研究. 湿法冶金, 1999(3): 1 https://www.cnki.com.cn/Article/CJFDTOTAL-SFYJ199903000.htm [17] Berger L M, Gruner W. Investigation of the effect of a nitrogen-containing atmosphere on the carbothermal reduction of titanium dioxide. Int J Refract Met Hard Mater, 2002, 20(3): 235 doi: 10.1016/S0263-4368(02)00019-7 [18] Fan M X, Peng J H, Chen H S, et al. A thermodynamics-based analysis of making titanium carbonitride through carbon thermal Reduction. J Kunming Univ Sci Technol Sic Technol, 2006, 31(5): 6 doi: 10.3969/j.issn.1007-855X.2006.05.002方民宪, 彭金辉, 陈厚生, 等. 碳热还原法制取碳氮化钛的热力学原理分析. 昆明理工大学学报(理工版), 2006, 31(5): 6 doi: 10.3969/j.issn.1007-855X.2006.05.002 [19] Pastor H. Titanium-carbonitride-based hard alloys for cutting tools. Mater Sci Eng A, 1988, 105(88): 401 -




 下载:
下载: