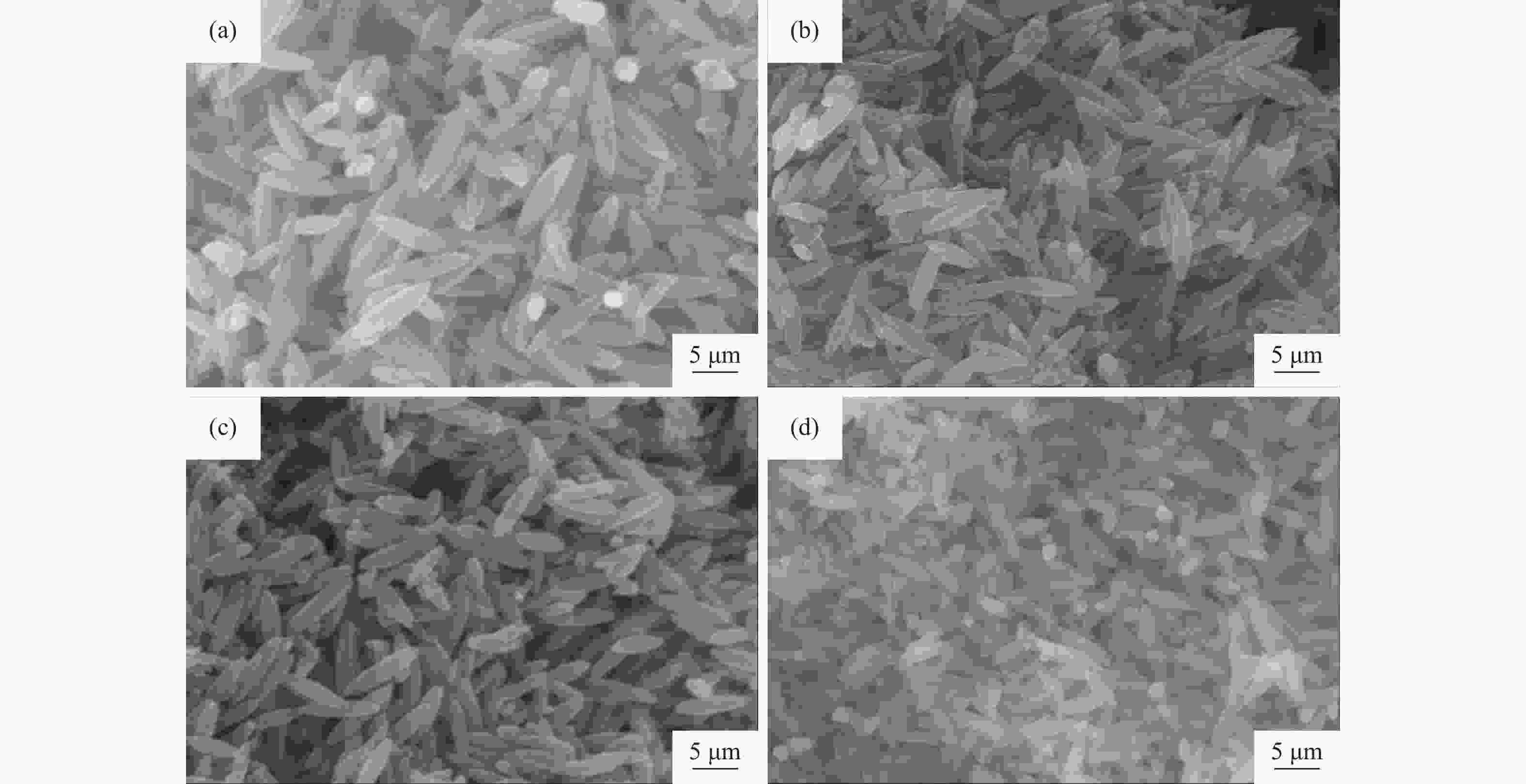
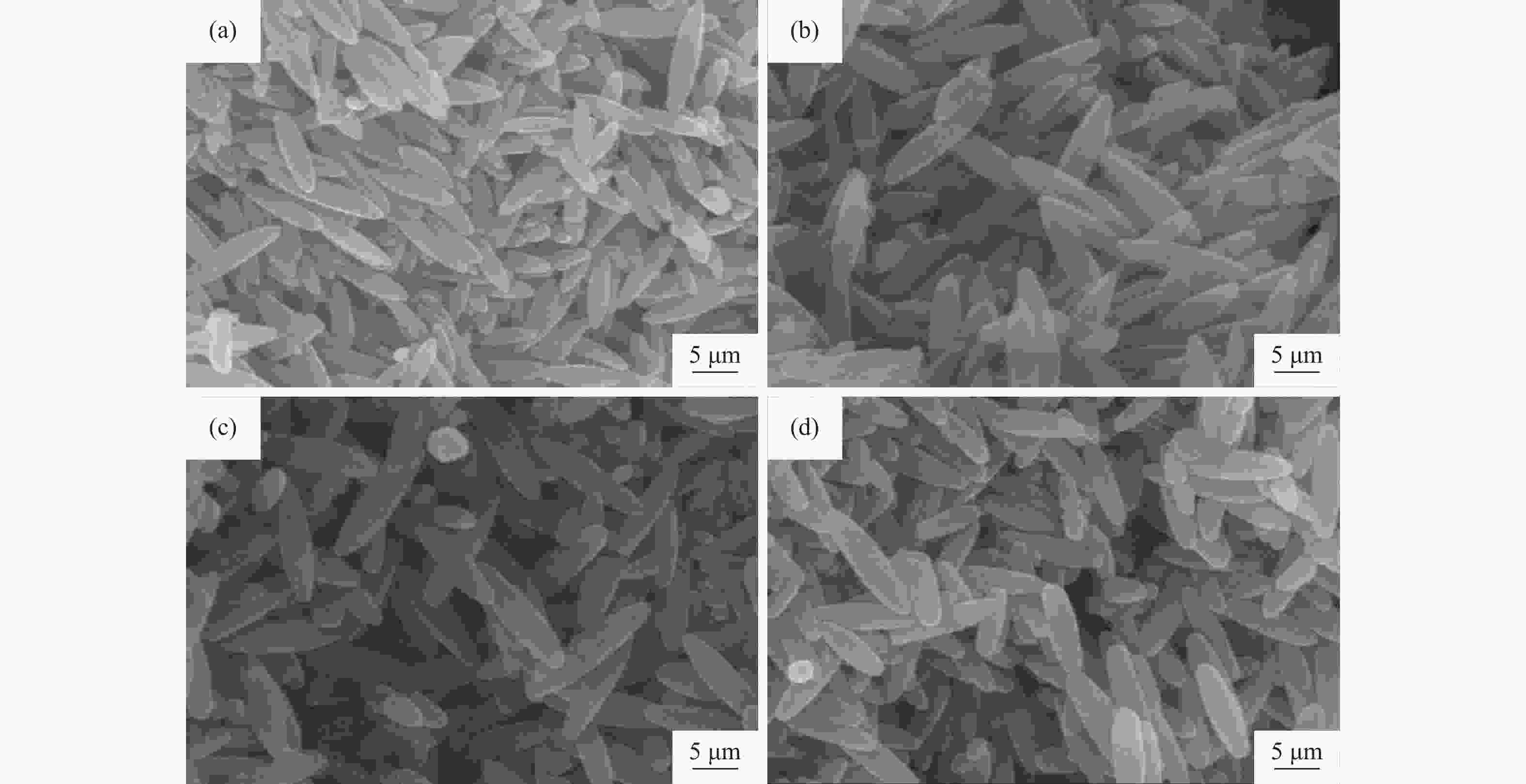
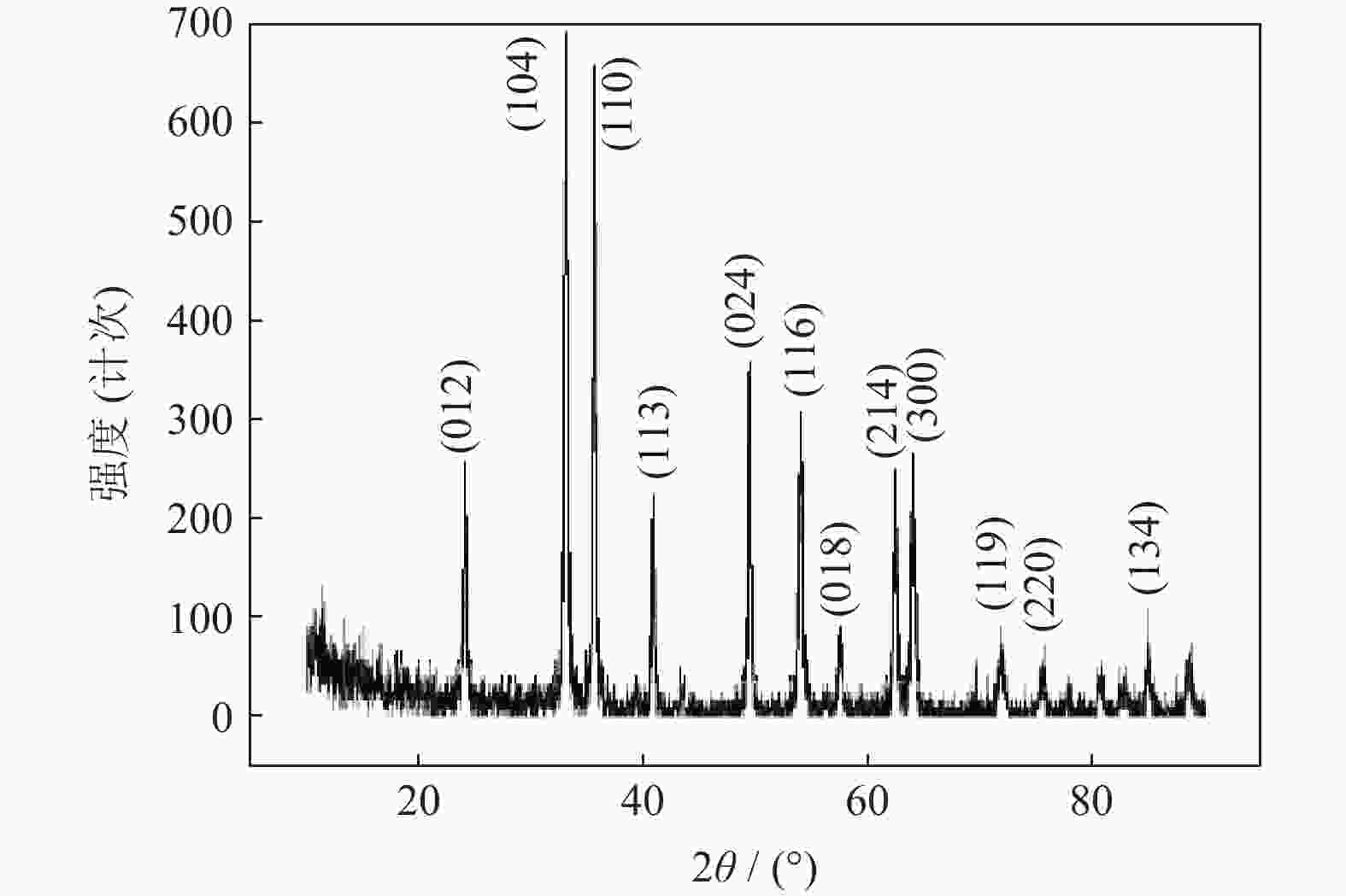
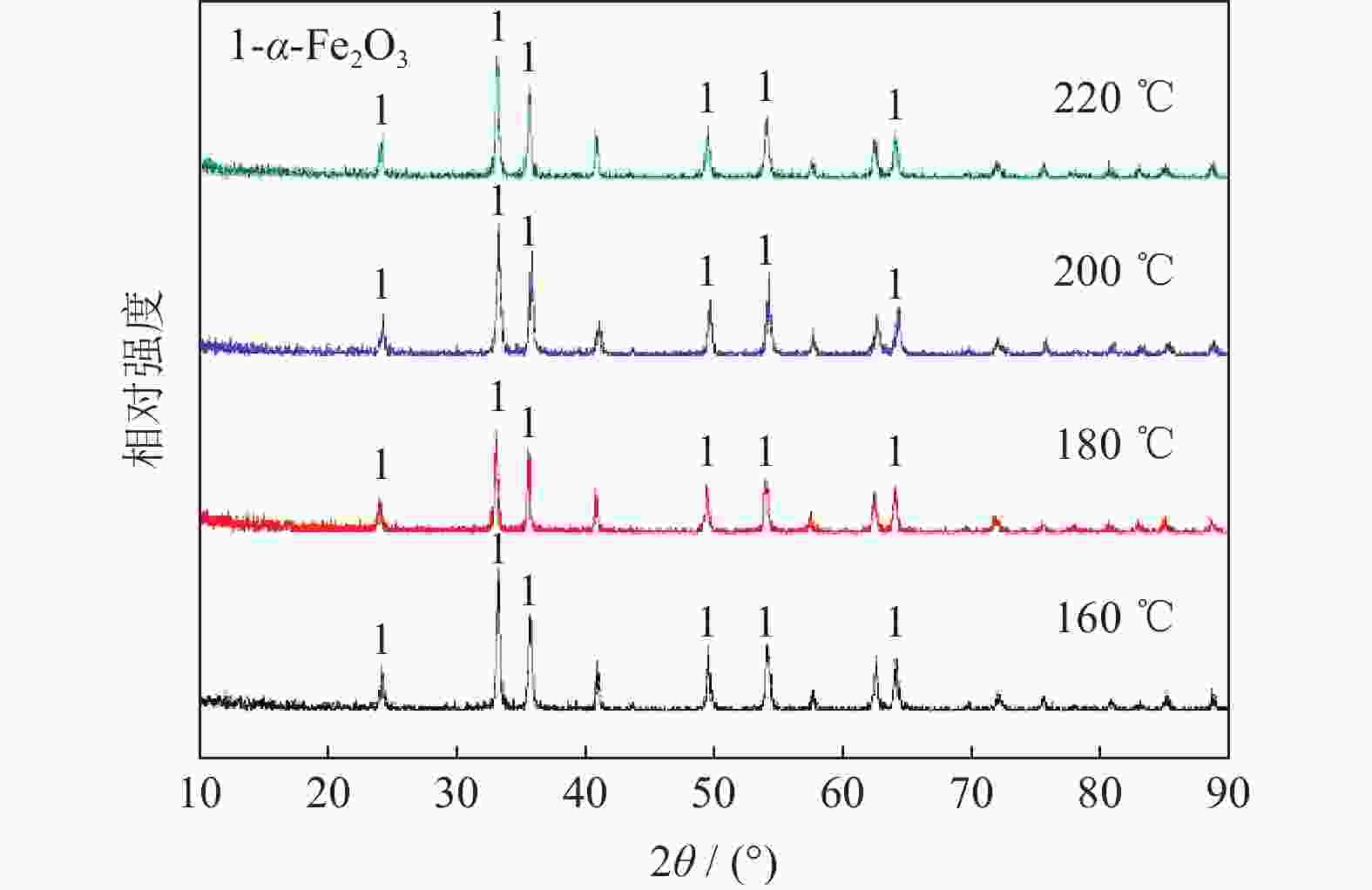
| [1] |
Zhang L S, Li H, Zhang H X, et al. Research progress on the application and preparation of nano-Fe3O4. Powder Metall Technol, 2019, 37(3): 233张立生, 李慧, 张汉鑫, 等. 纳米Fe3O4应用及制备工艺研究进展. 粉末冶金技术, 2019, 37(3): 233
|
| [2] |
Wang X P, Yu Y, Hu X F, et al. Preparation of oxide films by liquid phase deposition. J Funct Mater, 2000, 31(4): 341 doi: 10.3321/j.issn:1001-9731.2000.04.002王晓萍, 于云, 胡行方, 等. 液相沉积法制备氧化物薄膜. 功能材料, 2000, 31(4): 341 doi: 10.3321/j.issn:1001-9731.2000.04.002
|
| [3] |
Li F, Liu L, An Y, et al. Hydrothermal liquefaction of three kinds of starches into reducing sugars. J Cleaner Prod, 2016, 112(1): 1049
|
| [4] |
Qi L Y, Hao S, Li Y J. Synthesis of various morphological Ca0.75Er0.25MnO3 powders by hydrothermal method and their electrical properties. Res Chem Intermed, 2017, 43(11): 6589 doi: 10.1007/s11164-017-3006-4
|
| [5] |
Shi E W, Xia C T, Wang B G, et, al. Application and development of hydrothermal method. J Inorg Mater, 1996, 11(2): 193 doi: 10.3321/j.issn:1000-324X.1996.02.001施尔畏, 夏长泰, 王步国, 等. 水热法的应用与发展. 无机材料学报, 1996, 11(2): 193 doi: 10.3321/j.issn:1000-324X.1996.02.001
|
| [6] |
Zheng X F. Research progress in preparation of nano-oxides by hydrothermal method. Inorg Chem Ind, 2009, 41(8): 9 doi: 10.3969/j.issn.1006-4990.2009.08.003郑兴芳. 水热法制备纳米氧化物的研究进展. 无机盐工业, 2009, 41(8): 9 doi: 10.3969/j.issn.1006-4990.2009.08.003
|
| [7] |
Yu W G, Zhang T L, Zhang J G, et al. The preparation methods of magnetite nanoparticles and their morphology. Prog Chem, 2007, 19(6): 884 doi: 10.3321/j.issn:1005-281X.2007.06.006于文广, 张同来, 张建国, 等. 纳米四氧化三铁(Fe3O4)的制备和形貌. 化学进展, 2007, 19(6): 884 doi: 10.3321/j.issn:1005-281X.2007.06.006
|
| [8] |
Li C K, Qi H Z, Yan B. Chemical preparation and application of magnetic Fe3O4 nano-particles. Shanghai Met, 2009, 31(4): 54 doi: 10.3969/j.issn.1001-7208.2009.04.013李成魁, 祁红璋, 严彪. 磁性纳米四氧化三铁颗粒的化学制备及应用进展. 上海金属, 2009, 31(4): 54 doi: 10.3969/j.issn.1001-7208.2009.04.013
|
| [9] |
Xu Z C, Wu L L, Chen T. Research progress of direct-spinning based fabrication of carbon nanotube fibers. China Text Leader, 2019, 37(6): 67 doi: 10.3969/j.issn.1003-3025.2019.06.013徐子超, 吴丽莉, 陈廷. 直接气相沉积法制备碳纳米管纤维的研究进展. 纺织导报, 2019, 37(6): 67 doi: 10.3969/j.issn.1003-3025.2019.06.013
|
| [10] |
Zhuang X D, Li R X, Yu X H, et al. Preparation of lithium titanate electrode materials by solid phase method. Mater Rep, 2019, 33(16): 2654 doi: 10.11896/cldb.18060159庄晓东, 李荣兴, 俞小花, 等. 固相法制备钛酸锂电极材料. 材料导报, 2019, 33(16): 2654 doi: 10.11896/cldb.18060159
|
| [11] |
Song X M. Supercritical Method Preparation for Lithium Iron Phosphate Nanometer Material and Its Carbon-Coating Research[Dissertation]. Shanghai: Shanghai Jiao Tong University, 2012宋续明. 超临界法连续制备磷酸铁锂纳米正极材料及其碳包覆研究[学位论文]. 上海: 上海交通大学, 2012
|
| [12] |
Shao P H. Optimizing Synthesis of Iron Oxide Nano-Materials for Application in Photo-Fenton Degradation of Bisphenol S under Visible Light Irradiation[Dissertation]. Harbin: Harbin Institute of Technology, 2017邵鹏辉. 纳米氧化铁的优化制备及其可见光芬顿降解水中的双酚S[学位论文]. 哈尔滨: 哈尔滨工业大学, 2017
|
| [13] |
Jiao J G. The Controllable Synthesis and Study on Magnetic Properties of Irod Oxide-Based Nanorods and Nanoparticles[Dissertation]. Harbin: Harbin Institute of Technology, 2017焦建国. 氧化铁基纳米棒(颗粒)的可控合成与磁性能研究[学位论文]. 哈尔滨: 哈尔滨工业大学, 2017
|
| [14] |
Liu B, Zeng H C. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm. J Am Chem Soc, 2003, 125(15): 4430 doi: 10.1021/ja0299452
|
| [15] |
Du Y W, Li Z Y, Lu H X, et al. Study on the formation conditions of FeOOH. Acta Phys Sin, 1979, 28(5): 705 doi: 10.3321/j.issn:1000-3290.1979.05.012都有为, 李正宇, 陆怀先, 等. FeOOH生成条件的研究. 物理学报, 1979, 28(5): 705 doi: 10.3321/j.issn:1000-3290.1979.05.012
|



 下载:
下载: