Microstructure and electrochemical properties of 3D flower-like CoS anode materials used for lithium ion batteries synthesized by one-step hydrothermal method
-
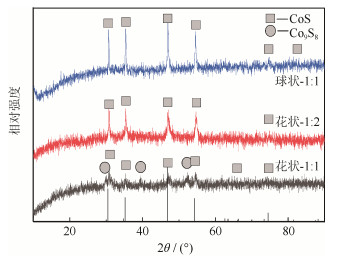
摘要: 以CoCl2·6H2O和硫脲(CH4N2S)为原料, 采用一步水热法, 通过改变钴硫摩尔比和添加表面活性剂制备出两种不同形貌(3D花状和球状)的硫化钴(CoS)锂离子电池负极材料。结果表明, 当钴硫摩尔比为1:1时, 在180℃下水热反应12 h可得到3D花状CoS负极材料, 其三维立体花状结构由纳米级层片组成; 当钴硫摩尔配比为1:1, 添加十六烷基三甲基溴化铵(CTAB), 在180℃下反应12 h可制得由小颗粒聚结成的球状CoS负极材料。在0.1C电流密度下, 3D花状CoS电池首次放电比容量为752 mAh·g-1, 并且具有良好的倍率性能; 在1C电流密度下, 经过200圈的循环测试后, 3D花状CoS电池仍有较高的放电比容量(185 mAh·g-1), 远高于球状CoS电池(118.6 mAh·g -1), 并且没有衰减的趋势。Abstract: Using CoCl2·6H2O and thiourea (CH4N2S) as the raw materials, the 3D flower-like and spherical cobalt sulfide (CoS) anode materials used for lithium ion batteries were synthesized by one-step hydrothermal method in this paper, through changing the molar ratio of Co to S and adding the surfactant. In the results, when the molar ratio of Co to S is 1:1 and the hydrothermal reaction is carried out at 180 fo℃ r 12 h, the obtained 3D flower-like CoS anode materials are composed of nano-scale layer structures. By adding the cetyl trimethyl ammonium bromide (CTAB) as surfactant, the globular cobalt sulfide anode materials formed by the agglomeration of small particles are obtained by hydrothermal reaction at 180 for 12 h℃ when the molar ratio of Co to S is 1:1. The first discharge capacity of 3D flower-like CoS anode materials is 752 mAh·g-1 at 0.1C, showing the good rate capability, and there is still high discharge capacity (185 mAh·g-1) after 200 cycles at 1C without the tendency of decay, which is much higher than that of the spherical CoS anode materials (118.6 mAh·g-1).
-
图 2 花状和球状硫化钴试样显微形貌:(a)和(b)花状-1:1;(c)和(d)花状-1:2;(e)和(f)球状-1:1;(g)高倍率花状-1:1
Figure 2. SEM images of flower-like and spherical cobalt sulfide samples: (a) and (b) flower-like sample-1:1; (c) and (d) flower-like sample-1:2; (e) and (f) spherical sample-1:1; (g) higher magnification of flower-like sample-1:1
表 1 硫化钴样品的比表面积和孔体积数值
Table 1. Specific surface area and pore volume of cobalt sulfide samples
试样 比表面积/ (m2·g‒1) 孔体积/ (mL·g‒1) 花状-1:1 15.3611 0.0593 花状-1:2 6.2693 0.0364 球状-1:1 4.1639 0.0180 表 2 CoS电极材料阻抗参数
Table 2. Impedance parameters of CoS electrode materials
试样 欧姆电阻/ Ω 传荷电阻/ Ω 阻抗总和/ Ω 花状-1:1 1.75 175.12 176.87 球状-1:1 9.17 377.67 386.84 花状-1:2 1.82 242.73 244.55 -
[1] Pasquariello D M, Kershaw R, Passaretti J D, et al. Low-temperature synthesis and properties of cobalt sulfide (Co9S8), nickel sulfide (Ni3S2), and iron sulfide (Fe7S8). Prep Inorg Chem, 1984, 15(25): 872 doi: 10.1002/chin.198425040 [2] Yuan C Z, Gao B, Su L H, et al. Electrochemically induced phase transformation and charge-storage mechanism of amorphous CoSx nanoparticles prepared by interface-hydrothermal method. J Electrochem Soc, 2009, 156(3): A199 doi: 10.1149/1.3065086 [3] Bao S J, Li Y B, Li C M, et al. Shape evolution and magnetic properties of cobalt sulfide. Cryst Growth Des, 2008, 8(10): 3745 doi: 10.1021/cg800381e [4] Liu S M. Synthesis and Electrochemical Properties of Transition Metal Sulfides and their Composite Materials[Dissertation]. Changchun: Changchun University of Science and Technology, 2014刘淑敏. 过渡金属硫化物及其复合材料的合成和电化学性能研究[学位论文]. 长春: 长春理工大学, 2014 [5] Wang Y M, Wu J J, Tang Y F, et al. Phase-controlled synthesis of cobalt sulfides for lithium ion batteries. ACS Appl Mater Interfaces, 2012, 4(8): 4246 doi: 10.1021/am300951f [6] Han W C. Composites of Graphene and Transition Metal Sulfides as Anode Materials for Lithium Ion Batteries[Dissertation]. Wuhu: Anhwei Normal University, 2016韩超伟. 石墨烯复合过渡金属硫化物作为锂离子电池负极材料的研究[学位论文]. 芜湖: 安徽师范大学, 2016 [7] Zhang C F, Wu H B, Guo Z P, et al. Facile synthesis of carbon-coated MoS2 nanorods with enhanced lithium storage properties. Electrochem Commun, 2012, 20: 7 doi: 10.1016/j.elecom.2012.03.039 [8] Zhou Y L, Yan D, Xu H Y, et al. Multiwalled carbon nanotube@a-C@Co9S8 nanocomposites: a high-capacity and long-life anode material for advanced lithium ion batteries. Nanoscale, 2015, 7(8): 3520 doi: 10.1039/C4NR07143C [9] Débart A, Dupont L, Patrice R, et al. Reactivity of transition metal (Co, Ni, Cu) sulphides versus lithium: the intriguing case of the copper sulphide. Solid State Sci, 2006, 8(6): 640 doi: 10.1016/j.solidstatesciences.2006.01.013 [10] Wang J, Ng S H, Wang G X, et al. Synthesis and characterization of nanosize cobalt sulfide for rechargeable lithium batteries. J Power Sources, 2006, 159(1): 287 doi: 10.1016/j.jpowsour.2006.04.092 [11] Gómez-Cámer J L, Martin F, Morales J, et al. Precipitation of CoS vs ceramic synthesis for improved performance in lithium cells. J Electrochem Soc, 2008, 155(3): A189 doi: 10.1149/1.2825137 [12] Wang Q H, Jiao L F, Han Y, et al. CoS2 hollow spheres: fabrication and their application in lithium-ion batteries. J Phys Chem C, 2011, 115(16): 8300 doi: 10.1021/jp111626a [13] Gu Y, Xu Y, Wang Y. Graphene-wrapped CoS nanoparticles for high-capacity lithium-ion storage. ACS Appl Mater Interfaces, 2013, 5(3): 801 doi: 10.1021/am3023652 [14] Seo J W, Jang J T, Park S W, et al. Two-dimensional SnS2 nanoplates with extraordinary high discharge capacity for lithium ion batteries. Adv Mater, 2010, 20(22): 4269 http://pubs.rsc.org/en/Content/OpenURL/50003391/C3EE42591F/107_1 [15] Fan Y, Shao H, Wang J, et al. Synthesis of foam-like freestanding Co3O4 nanosheets with enhanced electrochemical activities. Chem Commun, 2011, 47(12): 3469 doi: 10.1039/c0cc05383j [16] Zhao L, Tao F Q, Quan Z, et al. Bubble template synthesis of copper sulfide hollow spheres and their applications in lithium ion battery. Mater Lett, 2012, 68(1): 28 http://www.sciencedirect.com/science/article/pii/S0167577X11011384 [17] Du N, Zhang H, Chen J E, et al. Metal oxide and sulfide hollow spheres: layer-by-layer synthesis and their application in lithium-ion battery. J Phys Chem B, 2008, 112(47): 14836 doi: 10.1021/jp8065376 [18] Hu S, Chen W, Zhou J, et al. Preparation of carbon coated MoS2 flower-like nanostructure with self-assembled nanosheets as high-performance lithium-ion battery anodes. J Mater Chem A, 2014, 2(21): 7862 doi: 10.1039/c4ta01247j [19] Jin R C, Li G H, Zhang Z J, et al. Carbon coated flower like Bi2S3 grown on nickel foam as binder-free electrodes for electrochemical hydrogen and Li-ion storage capacities. Electrochim Acta, 2015, 173: 458 doi: 10.1016/j.electacta.2015.05.021 [20] Stoller M D, Park S J, Zhu Y W, et al. Graphene-based ultracapacitors. Nano Lett, 2008, 8(10): 3498 doi: 10.1021/nl802558y [21] Lee C G, Wei X D, Kysar J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science, 2008, 321(5887): 385 doi: 10.1126/science.1157996 [22] Aboulaich A, Lorret O, Boury B, et al. Surfactant-free organo-soluble silica-titania and silica nanoparticles. Chem Mater, 2009, 21(13): 2577 doi: 10.1021/cm900291p [23] Kim Y S, Goodenough J B. Lithium insertion into transition-metal monosulfides: tuning the position of the metal 4s band. J Phys Chem C, 2008, 112(38): 15060 doi: 10.1021/jp8038847 [24] Wang Q H, Jiao L F, Du H M, et al. Novel flower-like CoS hierarchitectures: one-pot synthesis and electrochemical properties. J Mater Chem, 2010, 21(2): 327 http://pubs.rsc.org/en/content/articlelanding/2011/jm/c0jm03121f [25] Qiu W D, Jiao J Q, Xia J, et al. A self-standing and flexible electrode of yolk-shell CoS2 spheres encapsulated with nitrogen-doped graphene for high-performance lithium-ion batteries. Chemistry, 2015, 21(11): 4359 doi: 10.1002/chem.201405821 [26] Xie J, Liu S Y, Cao G S, et al. Self-assembly of CoS2/graphene nanoarchitecture by a facile one-pot route and its improved electrochemical Li-storage properties. Nano Energy, 2013, 2(1): 49 doi: 10.1016/j.nanoen.2012.07.010 -




 下载:
下载: