-
摘要: 氮化铝因高导热和绝缘性得到广泛应用,目前全球氮化铝应用市场处于高速成长期,对氮化铝的需求也在持续增长。氮化铝粉末是制备氮化铝陶瓷的关键原料,其性质对后续制备的氮化铝陶瓷性能有决定性影响。本文整理对比了微米级与纳米级氮化铝粉末的制备方法并对未来氮化铝粉末制备的研究方向和发展趋势提出了展望。Abstract: Aluminum nitride has been widely applied for the high thermal conductivity and insulating properties. Nowadays, the global aluminum nitride application market is in the high growth stage as well as the demand for aluminum nitride is growing continuously. Aluminum nitride powders are the critical raw materials for the synthesis of aluminum nitride ceramics, and the properties of the aluminum nitride powders dominate the properties of the aluminum nitride ceramics. In the paper, the preparation methods of micrometer- and nanometer-sized aluminum nitride powders have been compared. Moreover, the future research directions and development trend of preparing aluminum nitride powders have been pointed out.
-
Key words:
- aluminum nitride /
- powder synthesis process /
- functional ceramics /
- nanopowders
-
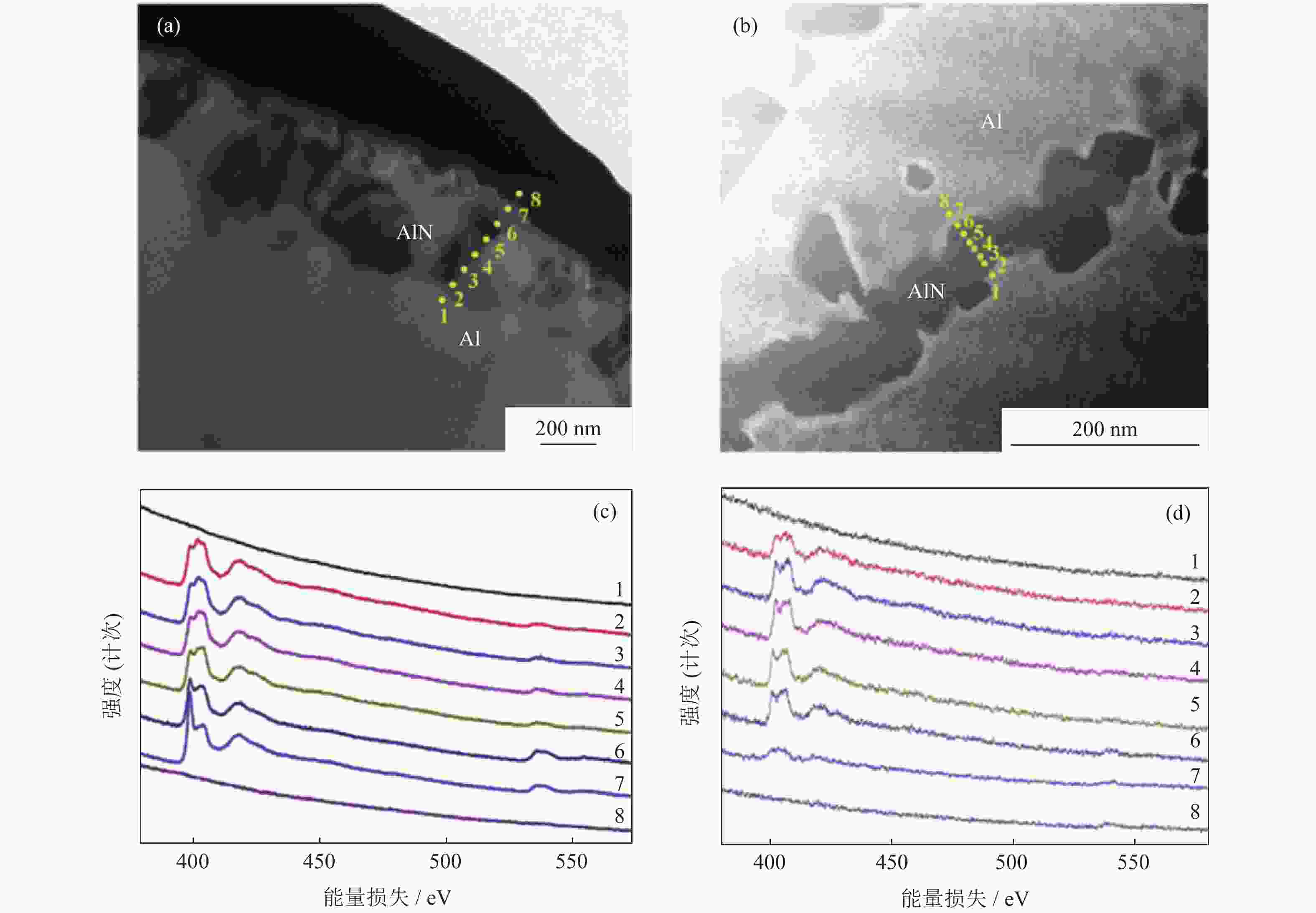
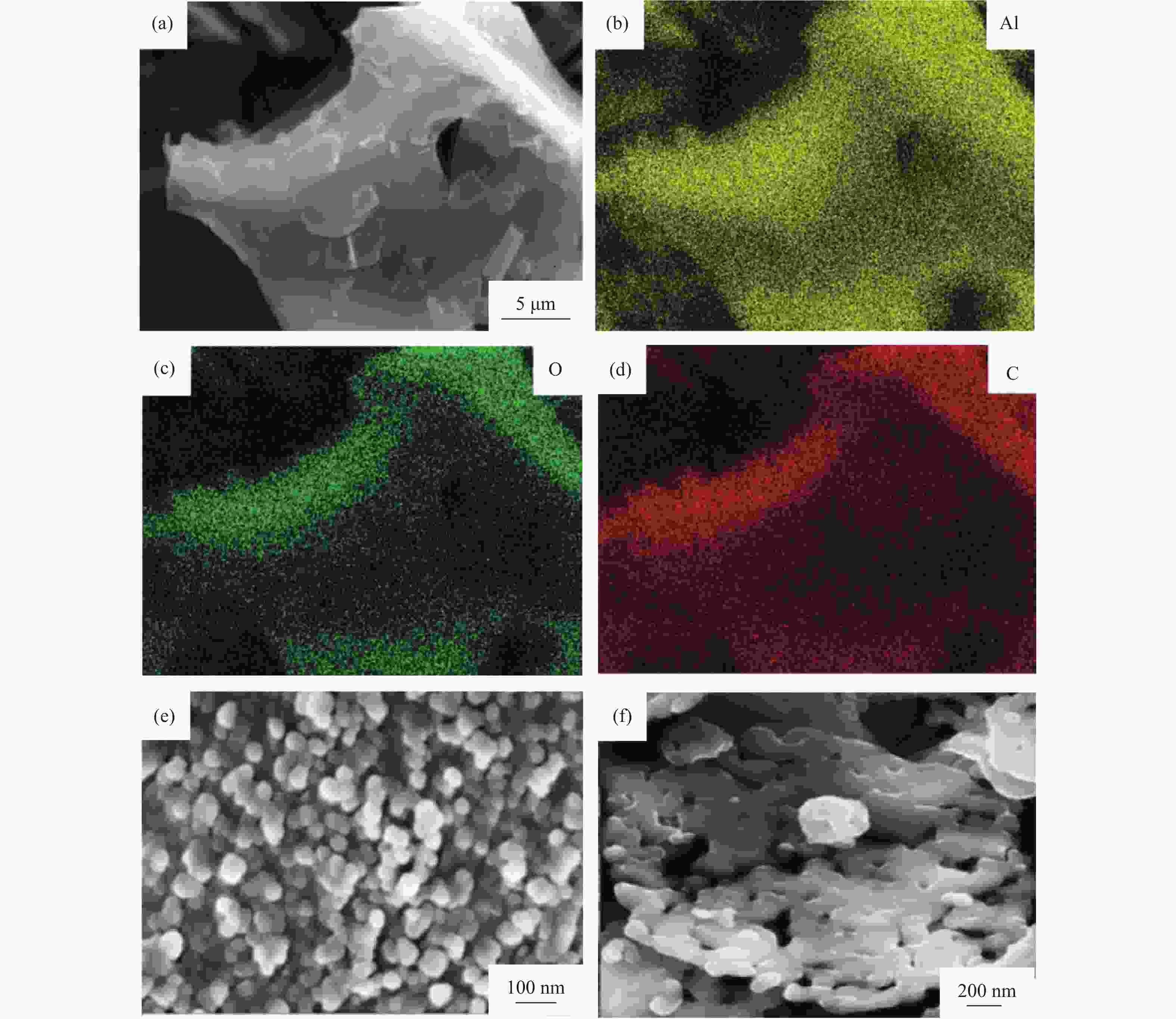
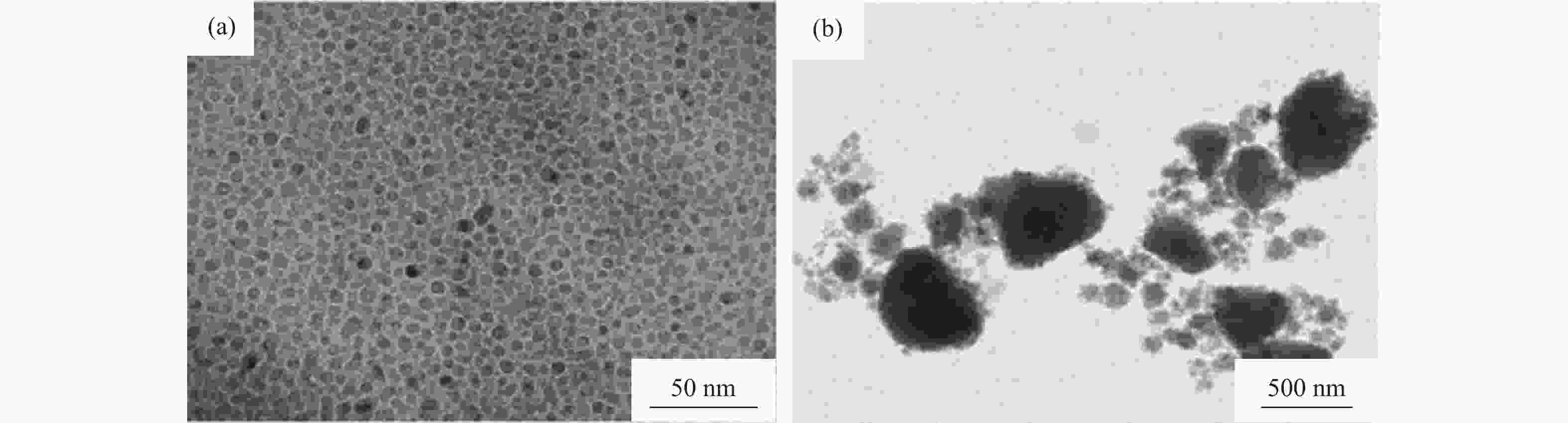
图 2 AlN颗粒透射电子显微形貌和电子能量损失谱[16]:(a)未加入碳的AlN颗粒显微形貌;(b)加入3%碳的AlN颗粒显微形貌;(c)未加入碳的AlN颗粒表面电子能量损失谱;(d)加入3%碳的AlN颗粒表面电子能量损失谱
Figure 2. Transmission electron microscope (TEM) images and electron energy loss spectroscopy (EELS) of AlN powders[16]: (a) TEM images without carbon additive; (b) TEM images with 3% carbon additive by mass; (c) EELS spectra without carbon additive; (d) EELS spectra with 3% carbon additive by mass
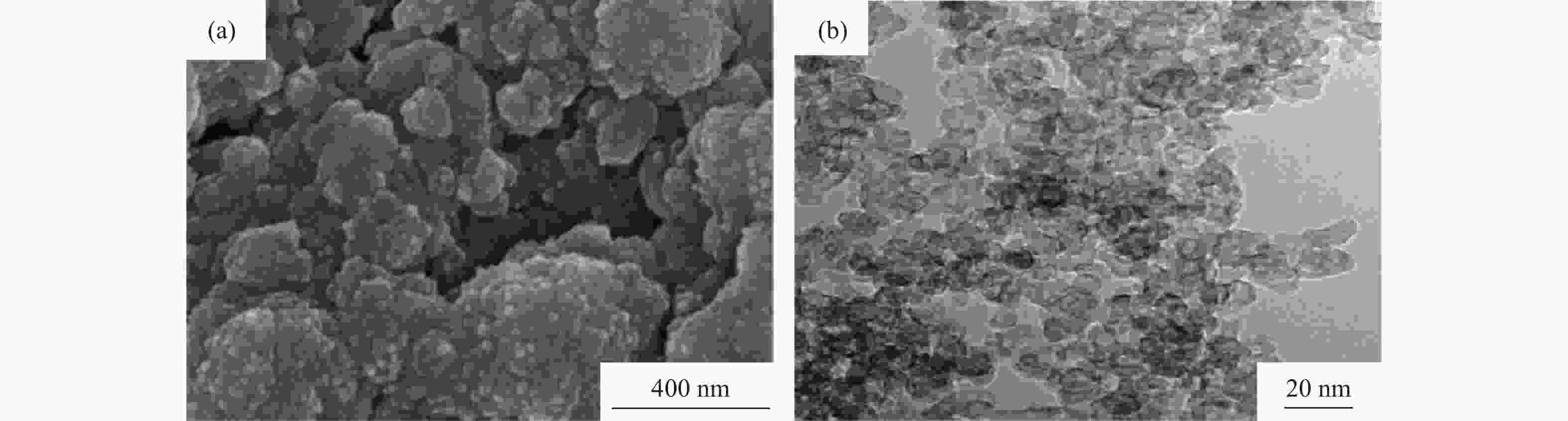
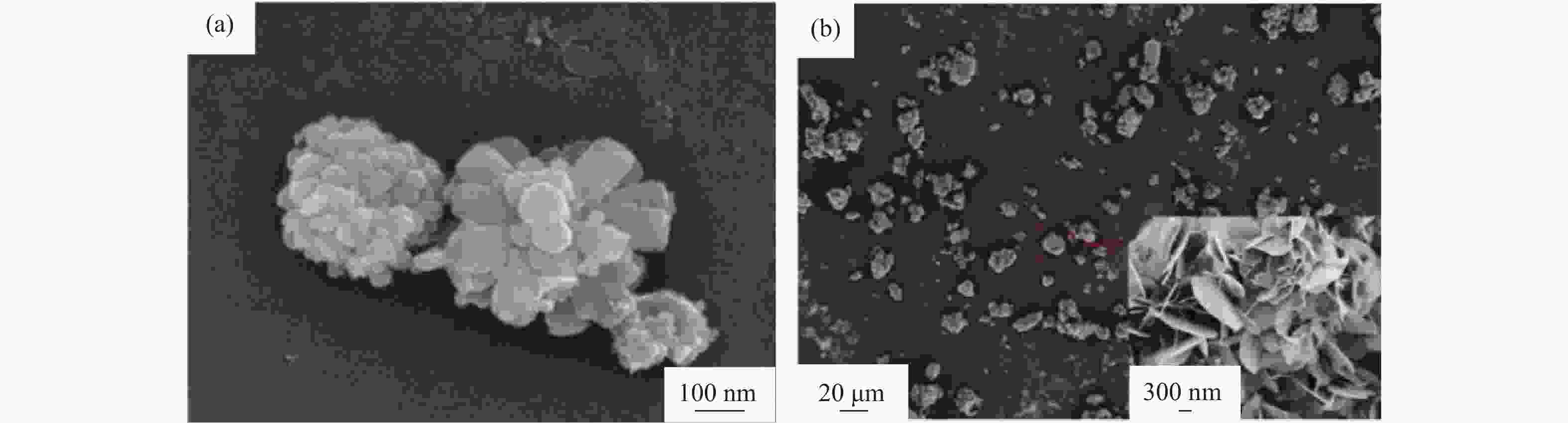
图 7 溶液燃烧合成AlN前驱物和合成纳米AlN粉末显微形貌和元素分布:(a)AlN前驱物显微形貌[23];(b)~(d)AlN前驱物元素分布[23];(e)纳米AlN粉末显微形貌[8];(f)具有六边形结构AlN粉末显微形貌[13]
Figure 7. Microstructure and element distribution of the AlN precursors and AlN powders prepared by solution combustion synthesis: (a) microstructure of the AlN precursors[23]; (b)~(d) element distribution of the AlN precursors[23]; (e) microstructure of the AlN powders[8]; (f) microstructure of the AlN powders with hexagonal structure[13]
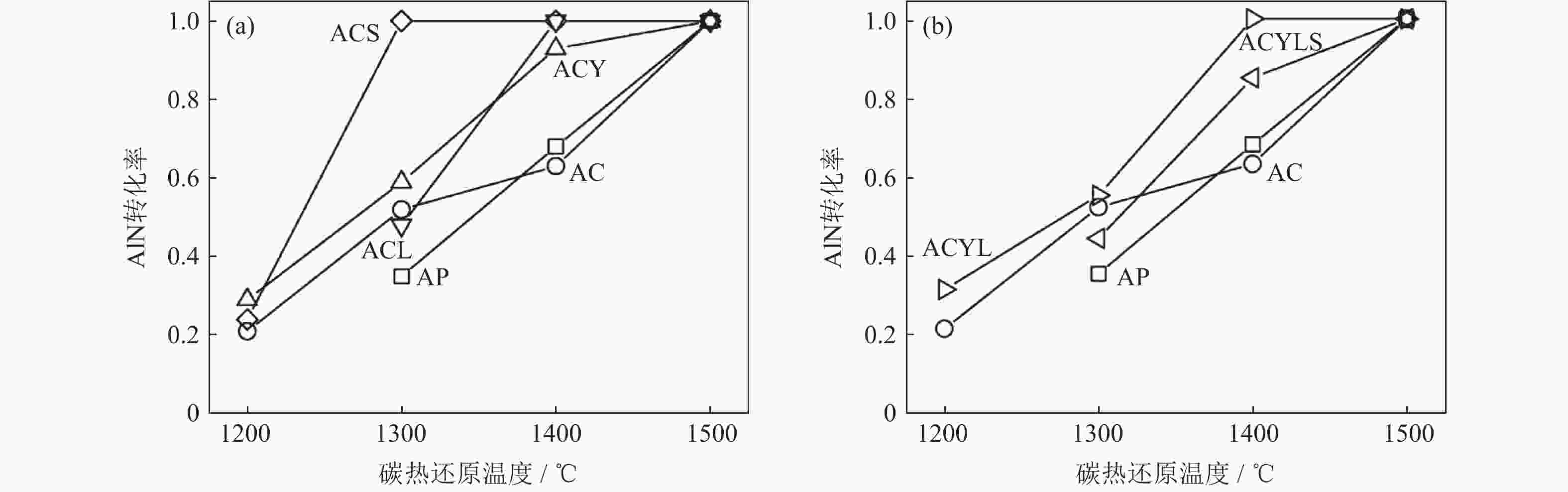
表 1 混粉过程中所用的原料及配比(质量分数)[46]
Table 1. Raw materials and compositions used in the powder mixing process[46]
% 试样编号 C Al2O3 CaF2 Y2O3 Li2Oa SrOa AP 32.0 68.0 — — — — AC 31.4 65.6 3.0 — — — ACY 30.4 64.6 3.0 2.00 — — ACL 30.4 64.6 3.0 — 2.0 — ACS 30.4 64.6 3.0 — — 2.00 ACYL 30.4 30.4 3.0 1.50 0.5 — ACYLS 30.4 30.4 3.0 0.75 0.5 0.75 注:a表示以碳酸盐形式添加 表 2 制备纳米AlN粉末的方法及特点
Table 2. Methods and characteristics of the nanometer AlN powder preparation
方法 特点 直接氮化法 纯度不高,转化率较低 湿化学法 原材料经济易得,能耗低,混合均匀 化学气相沉积法 产物纯度较高,但结晶度不高 高能物理辅助法 反应伴随较强的机械、光、热、电磁效应 机械化学法 室温球磨,反应时间长,残余应力较高 -
[1] Baik Y, Drew R A L. Aluminum nitride: Processing and applications. Key Eng Mater, 1996, 122-124: 553 doi: 10.4028/www.scientific.net/KEM.122-124.553 [2] Loughin S, French R H, Ching W Y, et al. Electronic structure of aluminum nitride: Theory and experiment. Appl Phys Lett, 1993, 63(9): 1182 doi: 10.1063/1.109764 [3] Yao Z Q, Li Y Q, Tang J X, et al. Growth and photoluminescence studies of AlN thin films with different orientation degrees. Diamond Relat Mater, 2008, 17(7-10): 1785 doi: 10.1016/j.diamond.2008.02.009 [4] Baskut S, Cinar A, Turan S. Directional properties and microstructures of spark plasma sintered aluminum nitride containing graphene platelets. J Eur Ceram Soc, 2017, 37(12): 3759 doi: 10.1016/j.jeurceramsoc.2017.03.032 [5] Enloe J H, Rice R W, Lau J W, et al. Microstructural effects on the thermal conductivity of polycrystalline aluminum nitride. J Am Ceram Soc, 1991, 74(9): 2214 doi: 10.1111/j.1151-2916.1991.tb08287.x [6] He Q, Qin M L, Huang M, et al. Synthesis of highly sinterable AlN nanopowders through sol-gel route by reduction-nitridation in ammonia. Ceram Int, 2019, 45(12): 14568 doi: 10.1016/j.ceramint.2019.04.174 [7] Chu A M, Qin M L, Rafi-ud-din, et al. Effect of urea on the size and morphology of AlN nanoparticles synthesized from combustion synthesis precursors. J Alloys Compd, 2012, 530: 144 doi: 10.1016/j.jallcom.2011.12.133 [8] Chu A M, Qin M L, Jia B R, et al. Effect of carbon source content on the carbothermal synthesis of AlN powders using a combustion synthesis precursor. Adv Mater Res, 2012, 554-556: 526 doi: 10.4028/www.scientific.net/AMR.554-556.526 [9] Chu A M, Qin M L, Rafi-ud-din, et al. Effect of aluminum source on the synthesis of AlN powders from combustion synthesis precursors. Mater Res Bull, 2012, 47(9): 2475 doi: 10.1016/j.materresbull.2012.05.014 [10] Wang L Y. Polymer network gel method for surperfine AlN powder preparation. Matec Web Conf, 2017, 109(6): 03006 [11] Wang H P, Yang Q H, Jia G H, et al. Influence of yttrium dopant on the synthesis of ultrafine AlN powders by CRN route from a sol–gel low temperature combustion precursor. Adv Powder Technol, 2014, 25(1): 450 doi: 10.1016/j.apt.2013.07.008 [12] Chu A M, Qin M L, Rafi-ud-din, et al. Citric acid-assisted combustion-carbothermal synthesis of well-distributed highly sinterable AlN nanopowders. J Am Ceram Soc, 2012, 95(8): 2510 doi: 10.1111/j.1551-2916.2012.05225.x [13] Wu H Y, Qin M L, Chu A M, et al. AlN powder synthesis by sodium fluoride-assisted carbothermal combustion. Ceram Int, 2014, 40(9): 14447 doi: 10.1016/j.ceramint.2014.07.014 [14] Zhang D, Liu F M, Cai L G, et al. Formation of novel core–shell and tadpole-like structures in the direct nitridation of aluminum powder by N2 and NH3. J Alloys Compd, 2013, 547: 91 doi: 10.1016/j.jallcom.2012.08.031 [15] Zhang Y H, Wang Q, Qu Z X, et al. Effect of additive Zn on the synthesis of AlN powders by direct nitridation. Rare Met Mater Eng, 2014, 43(7): 1727张耀辉, 王群, 瞿志学, 等. Zn元素对直接氮化法制备AlN粉体的影响. 稀有金属材料与工程, 2014, 43(7): 1727 [16] Lee K B, Kim Y H, Choi H J, et al. Effect of carbon on the nitridation behavior of aluminum powder. J Alloys Compd, 2016, 689: 218 doi: 10.1016/j.jallcom.2016.07.109 [17] Mackenzie M, Craven A J. Quantifying the oxidation of AlN using electron energy loss spectroscopy. J Phys D, 2000, 33(14): 1647 doi: 10.1088/0022-3727/33/14/303 [18] Chung S L, Lai C H. Combustion synthesis of aluminum nitride: A review. Key Eng Mater, 2012, 521(11): 101 [19] Lu H F. Study on Preparation and Injection Molding of Aluminum Nitride Powder [Dissertation]. Beijing: University of Science and Technology Beijing, 2020鲁慧峰. 氮化铝粉末制备及注射成形研究[学位论文]. 北京: 北京科技大学, 2020 [20] Pee J H, Park J C, Hwang K T, et al. Properties of AlN powder synthesized by self-propagating high temperature synthesis process. Key Eng Mater, 2010, 434-435: 834 doi: 10.4028/www.scientific.net/KEM.434-435.834 [21] Lin C N, Chung S L. Combustion synthesis method for synthesis of aluminum nitride powder using aluminum containers. J Mater Res, 2001, 16(12): 3518 doi: 10.1557/JMR.2001.0483 [22] Liu Z J, Wang W C, Yang D Z, et al. Synthesis of nano-size AlN powders by carbothermal reduction from plasma-assisted ball milling precursor. Plasma Sci Technol, 2016, 18(7): 759 doi: 10.1088/1009-0630/18/7/10 [23] He Q, Qin M L, Huang M, et al. Mechanism and kinetics of combustion-carbothermal synthesis of AlN nanopowders. Ceram Int, 2017, 43(12): 8755 doi: 10.1016/j.ceramint.2017.04.006 [24] Hideaki C, Jun F, Yamato H, et al. Kinetics of microwave synthesis of AlN by carbothermal-reduction-nitridation at low temperature. J Am Ceram Soc, 2018, 101(11): 4905 doi: 10.1111/jace.15903 [25] Kim J K, Jung W S. Nitridation of δ-alumina to aluminum nitride under a flow of ammonia and its mechanism. J Ceram Soc Jpn, 2011, 119(1389): 351 doi: 10.2109/jcersj2.119.351 [26] Ognjanović S M, Winterer M. Optimizing particle characteristics of nanocrystalline aluminum nitride. Powder Technol, 2017, 326: 488 [27] Pee J H, Park J C, Hwang K T, et al. Synthesis of an aluminum nitride–yttria (AlN–Y2O3) composite from nano-sized porous AlN and YCl3. Res Chem Intermed, 2010, 36(6-7): 801 doi: 10.1007/s11164-010-0184-8 [28] Lee S H, Yi J H, Kim J H, et al. Preparation of nanometer AlN powders by combining spray pyrolysis with carbothermal reduction and nitridation. Ceram Int, 2011, 37(6): 1967 doi: 10.1016/j.ceramint.2011.03.052 [29] Li L, Ni G H, Zhao Y J, et al. Synthesis of nano-AlN powders from Al wire by arc plasma at atmospheric pressure. Ceram Int, 2018, 44(17): 21810 doi: 10.1016/j.ceramint.2018.08.284 [30] Gao X, Chen P W, Wang X G, et al. Production of AlN nanopowders by electrical wire explosion in liquid nitrogen. Mater Sci Forum, 2018, 910: 46 doi: 10.4028/www.scientific.net/MSF.910.46 [31] Wang S, Wang W C, Yang D Z, et al. Direct synthesis of AlN nano powder by dielectric barrier discharge plasma assisted high-energy ball milling. J Mater Sci Mater Electron, 2016, 27(8): 8518 doi: 10.1007/s10854-016-4868-8 [32] Caballero E S, Cintas J, Cuevas F G, et al. A new method for synthetizing nanocrystalline aluminium nitride via a solid-gas direct reaction. Powder Technol, 2016, 287: 341 doi: 10.1016/j.powtec.2015.10.023 [33] Rounaghi S A, Kiani Rashid A R, Eshghi H, et al. Formation of nanocrystalline h-AlN during mechanochemical decomposition of melamine in the presence of metallic aluminum. J Solid State Chem, 2012, 190: 8 doi: 10.1016/j.jssc.2012.01.005 [34] Rounaghi S A, Eshghi H, Kiani Rashid A R, et al. Synthesis of nanostructured AlN by solid state reaction of Al and diaminomaleonitrile. J Solid State Chem, 2013, 198: 542 doi: 10.1016/j.jssc.2012.11.018 [35] Borovinskaya I P, Merzhanov A G, Novikov N P, et al. Gasless combustion of mixtures of powder transition metals with boron. Combust Explos Shock Waves, 1974, 10(1): 2 doi: 10.1007/BF01463777 [36] Merzhanov A G, Karyuk G G, Borovinskaya I P, et al. Titanium carbide produced by self-propagating high-temperature synthesis-Valuable abrasive material. Sov Powder Metall Met Ceram, 1981, 20(10): 709 doi: 10.1007/BF00791050 [37] Shim G, Park J S, Cho S W. Combustion synthesis of AlN with melamine as an additive. J Mater Res, 2006, 21(3): 747 doi: 10.1557/jmr.2006.0093 [38] Qiao L, Chen S W, Zheng J W, et al. Preparation and formation mechanism of aluminum nitride ceramic particles from large aluminum powder by self-propagating high temperature synthesis. Adv Powder Technol, 2015, 26(3): 830 doi: 10.1016/j.apt.2015.02.007 [39] Sun S Y, Ge Y Y, Wang Q, et al. Combustion synthesis of fine aluminum nitride powders under micropositive N2 pressure with water additive. J Am Ceram Soc, 2019, 102(3): 914 [40] Hiranaka A, Yi X M, Saito G, et al. Effects of Al particle size and nitrogen pressure on AlN combustion synthesis. Ceram Int, 2017, 43(13): 9872 doi: 10.1016/j.ceramint.2017.04.170 [41] Liu J P, Zhang H. Effects of NH4Cl on the synthesis of aluminum nitride by the spontaneous combustion of mechanically activated aluminium powder. Electron Compon Mater, 2011, 30(1): 8 doi: 10.3969/j.issn.1001-2028.2011.01.003刘建平, 张晖. NH4Cl对机械活化Al粉燃烧合成AlN的控制. 电子元件与材料, 2011, 30(1): 8 doi: 10.3969/j.issn.1001-2028.2011.01.003 [42] Xie X, Sui Y, Huang X Y, et al. Synthesis of AlN by direct combustion of Mg‒Al alloy powder. J Inorg Mater, 2019, 34(4): 439 doi: 10.15541/jim20180244谢晓, 隋颖, 黄晓昱, 等. 镁铝合金直接燃烧法合成AlN晶体. 无机材料学报, 2019, 34(4): 439 doi: 10.15541/jim20180244 [43] Hirai S, Miwa T, Iwata T, et al. Formation of AlN by carbothermic reduction of Al2O3 in a flowing N2 atmosphere. J Jpn Inst Met, 1989, 53: 1035 doi: 10.2320/jinstmet1952.53.10_1035 [44] Jung W S. Conversion of alumina to aluminum nitride using polymeric carbon nitride as a nitridation reagent. J Am Ceram Soc, 2016, 99(5): 1520 doi: 10.1111/jace.14152 [45] Jung W S. Effect of NiS addition on nitridation of alumina to aluminum nitride using polymeric carbon nitride as a nitridation reagent. Ceram Int, 2016, 42(13): 14716 doi: 10.1016/j.ceramint.2016.06.097 [46] André L M, Yoshimura H N. Low-temperature synthesis of AlN powder with multicomponent additive systems by carbothermal reduction–nitridation method. Mater Res Bull, 2010, 45(6): 733 doi: 10.1016/j.materresbull.2010.02.012 [47] Yan G N, Deng X Y, Lin J Z. The research of high-thermal-conductive aluminum nitride substrate in airport power electronics. Printed Circuit Inf, 2017, 25(10): 32 doi: 10.3969/j.issn.1009-0096.2017.10.006严光能, 邓先友, 林金堵. 高导热氮化铝基板在航空工业的应用研究. 印制电路信息, 2017, 25(10): 32 doi: 10.3969/j.issn.1009-0096.2017.10.006 [48] Dehkordi E N, Samim Banihashemi H R, Naghizadeh R, et al. Synthesis of aluminum nitride in a coke-calcium reduction bed using nitrogen in air. Int J Miner Metall Mater, 2015, 22(9): 972 doi: 10.1007/s12613-015-1157-0 [49] Zheng S Y, Chen J, Pan W. Compositing by wet-chemical method and its application. Mater Rev, 2000, 14(9): 25 doi: 10.3321/j.issn:1005-023X.2000.09.009郑仕远, 陈健, 潘伟. 湿化学方法合成及应用. 材料导报, 2000, 14(9): 25 doi: 10.3321/j.issn:1005-023X.2000.09.009 [50] Wei Y N, Wei H Y, Zhao D M, et al. Synthesis of aluminum nitride ultrafine powder via carbon thermal reduction nitridation process based on non-hydrolytic sol‒gel method. J Funct Mater, 2013, 44(17): 2546 doi: 10.3969/j.issn.1001-9731.2013.17.025魏颖娜, 魏恒勇, 赵冬梅, 等. 基于非水解sol‒gel法的碳热还原氮化合成氮化铝超细粉体. 功能材料, 2013, 44(17): 2546 doi: 10.3969/j.issn.1001-9731.2013.17.025 [51] Cheng Y L, Huang X, Xi X, et al. The effect of the urea content on the properties of nano-size AlN powders synthesized by a wet chemical method. Ceram Int, 2018, 44(5): 5774 doi: 10.1016/j.ceramint.2017.11.146 [52] Li Z X, Hao L C, Zhang J F, et al. Preparation of aluminum nitride nanomaterials by precursor method. J Chin Ceram Soc, 2020, 48(6): 787李紫璇, 郝留成, 张建飞, 等. 前驱体法合成氮化铝纳米材料及其生长机制. 硅酸盐学报, 2020, 48(6): 787 [53] Rounaghi S A, Vanpoucke D E P, Eshghi H, et al. A combined experimental and theoretical investigation of the Al-Melamine reactive milling system: A mechanistic study towards AlN-based ceramics. J Alloys Compd, 2017, 729: 240 doi: 10.1016/j.jallcom.2017.09.168 [54] Zhang W, Li Z, Zhang D. Synthesizing AlN powder by mechanochemical reaction between aluminum and melamine. J Mater Res, 2010, 25(3): 464 doi: 10.1557/JMR.2010.0076 [55] Liu Z J, Wang W C, Yang D Z, et al. In situ synthesis of AlN nanoparticles by solid state reaction from plasma assisted ball milling Al and diaminomaleonitrile mixture. Ceram Int, 2015, 42(2): 3411 -



 下载:
下载: