Preparation of ultrafine Mo powders by MoO3 pre-reduction with insufficient carbon and hydrogen deep reduction
-
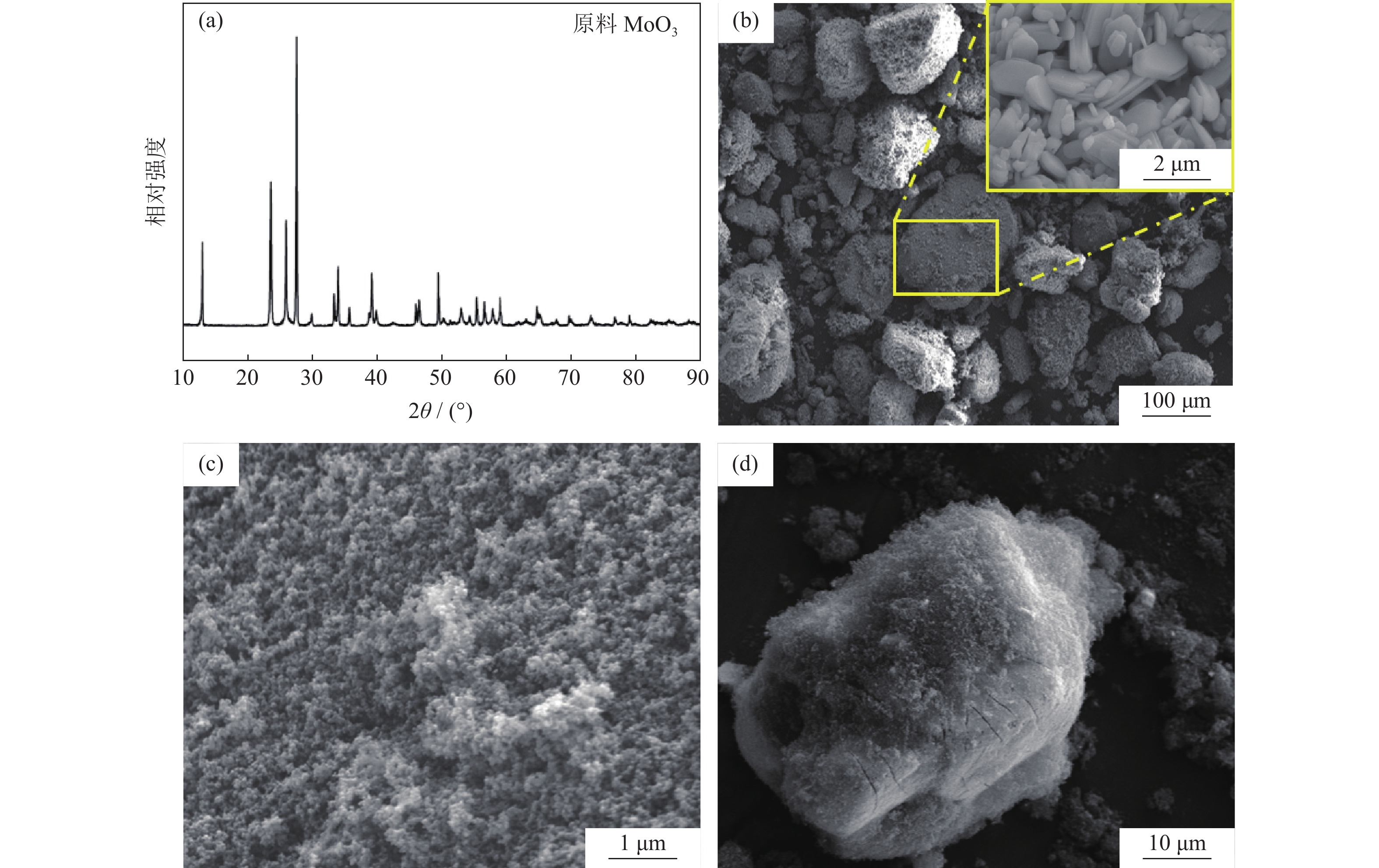
摘要: 以炭黑为还原剂还原MoO3制备存在少量MoO2的预还原Mo粉,然后对预还原Mo粉进行氢气深还原,成功制备出平均粒径为99~190 nm的超细钼粉,研究了碳热还原温度对Mo粉平均粒度和残碳量的影响。结果表明,在同一还原温度下,当C/MoO3摩尔比从2.0增加到2.1时,产物的粒径变化很小。碳热还原温度对产物粒径和纯度有显著影响。当C/MoO3摩尔比为2.1时,还原温度从950 ℃增加到1150 ℃,氢还原后钼粉的平均粒径从100 nm增加到190 nm,且残碳量(质量分数)由0.030%降低到0.009%。Abstract: The pre-reduction molybdenum powders containing minor amount of MoO2 were prepared by the carbothermic reduction of MoO3, using the carbon black as the reducing agent, then the obtained pre-reduction molybdenum powders were deep reduced by hydrogen to finally prepare the ultrafine molybdenum powders with the average particle size of 99 nm to 190 nm. The effects of the carbothermic reduction temperature on the particle size and the residual carbon content by mass of the ultrafine molybdenum powders were studied. In the results, with the increase of C/MoO3 molar ratio from 2.0 to 2.1, the particle sizes of the ultrafine molybdenum powder products change little at the same reduction temperature. But the carbothermal reduction temperature shows a significant effect on the particle size and purity of products. When the C/MoO3 molar ratio is 2.1, with the increase of reduction temperature from 950 ℃ to 1150 ℃, the average particle sizes of products increase from 100 nm to 190 nm, and the content of the residual carbon by mass decreases from 0.030% to 0.009% after the hydrogen reduction.
-
Key words:
- ultrafine molybdenum powders /
- carbothermic reduction /
- hydrogen reduction /
- nucleation /
- growth
-
图 4 不同温度和不同C/MoO3摩尔比的碳热还原产物显微形貌:(a)950 ℃,2.0;(b)950 ℃,2.1;(c)950 ℃,2.2;(d)1000 ℃,2.0;(e)1000 ℃,2.1;(f)1050 ℃,2.0;(g)1050 ℃,2.1;(h)1100 ℃,2.1;(i)1150 ℃,2.1
Figure 4. SEM images of the carbothermic reduction products at different temperatures and C/MoO3 ratios by mole: (a) 950 ℃, 2.0; (b) 950 ℃, 2.1; (c) 950 ℃, 2.2; (d) 1000 ℃, 2.0; (e) 1000 ℃, 2.1; (f) 1050 ℃, 2.0; (g) 1050 ℃, 2.1; (h) 1100 ℃, 2.1; (i) 1150 ℃, 2.1
-
[1] Gu S, Qin M, Zhang H, et al. Preparation of Mo nanopowders through hydrogen reduction of a combustion synthesized foam-like MoO2 precursor. Int J Refract Met Hard Mater, 2018, 76: 90 doi: 10.1016/j.ijrmhm.2018.05.015 [2] Wang L, Zhang G H, Wang J S, et al. Study on hydrogen reduction of ultrafine MoO2 to produce ultrafine Mo. J Phys Chem C, 2016, 120(7): 4097 doi: 10.1021/acs.jpcc.5b11394 [3] Sun G D, Zhang G H, Jiao S Q, et al. Shape-controlled synthesis of ultrafine molybdenum crystals via salt-assisted reduction of MoO2 with H2. J Phys Chem C, 2018, 122(18): 10231 doi: 10.1021/acs.jpcc.8b01236 [4] Ku J G, Oh J M, Kwon H, et al. High-temperature hydrogen-reduction process for the preparation of low-oxygen Mo powder from MoO3. Int J Hydrogen Energy, 2017, 42(4): 2139 doi: 10.1016/j.ijhydene.2016.09.004 [5] Zhang G J, Sun Y J, Niu R M, et al. Microstructure and strengthening mechanism of oxide lanthanum dispersion strengthened molybdenum alloy. Adv Eng Mater, 2004, 6(12): 943 doi: 10.1002/adem.200400072 [6] Kang J, Sun X, Deng K, et al. High strength Mg-9Al serial alloy processed by slow extrusion. Mater Sci Eng A, 2017, 697: 211 doi: 10.1016/j.msea.2017.05.017 [7] Kim G S, Lee Y J, Kim D G, et al. Consolidation behavior of Mo powder fabricated from milled Mo oxide by hydrogen-reduction. J Alloys Compd, 2008, 454(1-2): 327 doi: 10.1016/j.jallcom.2006.12.039 [8] Dang J, Zhang G H, Chou K C. Study on kinetics of hydrogen reduction of MoO2. Int J Refract Met Hard Mater, 2013, 41: 356 doi: 10.1016/j.ijrmhm.2013.05.009 [9] Bolitschek J, Luidold S, O'Sullivan M. A study of the impact of reduction conditions on molybdenum morphology. Int J Refract Met Hard Mater, 2018, 71: 325 doi: 10.1016/j.ijrmhm.2017.11.037 [10] Millner T, Neugebauer J. Volatility of the oxides of tungsten and molybdenum in the presence of water vapour. Nature, 1949, 163(4146): 601 [11] Lenz M, Gruehn R. Developments in measuring and calculating chemical vapor transport phenomena demonstrated on Cr, Mo, W, and their compounds. Chem Rev, 1997, 97(8): 2967 doi: 10.1021/cr940313a [12] Schulmeyer W V, Ortner H M. Mechanisms of the hydrogen reduction of molybdenum oxides. Int J Refract Met Hard Mater, 2002, 20(4): 261 doi: 10.1016/S0263-4368(02)00029-X [13] Sun G D, Zhang G H. Novel pathway to prepare Mo nanopowder via hydrogen reduction of MoO2 containing Mo nanoseeds produced by reducing MoO3 with carbon black. JOM, 2020, 72: 347 doi: 10.1007/s11837-019-03445-4 [14] Lassner E, Schubert W D. Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds. Vienna: Vienna University of Technology, 1999 [15] L’ vov B V. Mechanism of carbothermal reduction of iron, cobalt, nickel and copper oxides. Thermochim Acta, 2000, 360(2): 109 doi: 10.1016/S0040-6031(00)00540-2 [16] Venables D S, Brown M E. Reduction of tungsten oxides with carbon. Part 1: Thermal analyses. Thermochim Acta, 1996, 282-283: 251 doi: 10.1016/0040-6031(95)02814-5 [17] Wang L, Zhang G H, Sun Y J, et al. Preparation of ultrafine β-MoO3 from industrial grade MoO3 powder by the method of sublimation. J Phys Chem C, 2016, 120(35): 19821 doi: 10.1021/acs.jpcc.6b05982 [18] Wang L, Zhang G H, Chou K C. Mechanism and kinetic study of hydrogen reduction of ultra-fine spherical MoO3 to MoO2. Int J Refract Met Hard Mater, 2016, 54: 342 doi: 10.1016/j.ijrmhm.2015.09.003 [19] Wang L, Zhang G H, Chou K C. Synthesis of nanocrystalline molybdenum powder by hydrogen reduction of industrial grade MoO3. Int J Refract Met Hard Mater, 2016, 59: 100 doi: 10.1016/j.ijrmhm.2016.06.001 [20] Zhang G H, Li J J, Wang L, et al. Effects of R2CO3 (R= Li, Na and K) on the reduction of MoO2 to Mo by hydrogen. Int J Refract Met Hard Mater, 2017, 69: 180 doi: 10.1016/j.ijrmhm.2017.08.014 [21] Sun G D, Wang K F, Ji X P, et al. Preparation of ultrafine/nano Mo particles via NaCl-assisted hydrogen reduction of different-sized MoO2 powders. Int J Refract Met Hard Mater, 2019, 80: 243 doi: 10.1016/j.ijrmhm.2019.01.020 [22] Sun G D, Zhang G H, Chou K C. Preparation of Mo nanoparticles through hydrogen reduction of commercial MoO2 with the assistance of molten salt. Int J Refract Met Hard Mater, 2019, 78: 68 doi: 10.1016/j.ijrmhm.2018.08.014 [23] Zhang Y, Jiao S Q, Chou K C, et al. Size-controlled synthesis of Mo powders via hydrogen reduction of MoO2 powders with the assistance of Mo nuclei. Int J Hydrogen Energy, 2020, 45(3): 1435 doi: 10.1016/j.ijhydene.2019.11.008 [24] Wang D H, Sun G D, Zhang G H. Preparation of ultrafine Mo powders via carbothermic pre-reduction of molybdenum oxide and deep reduction by hydrogen. Int J Refract Met Hard Mater, 2018, 75: 70 doi: 10.1016/j.ijrmhm.2018.04.002 [25] Sun G D, Zhang G H, Ji X P, et al. Size-controlled synthesis of nano Mo powders via reduction of commercial MoO3 with carbon black and hydrogen. Int J Refract Met Hard Mater, 2019, 80: 11 doi: 10.1016/j.ijrmhm.2018.12.019 -




 下载:
下载: