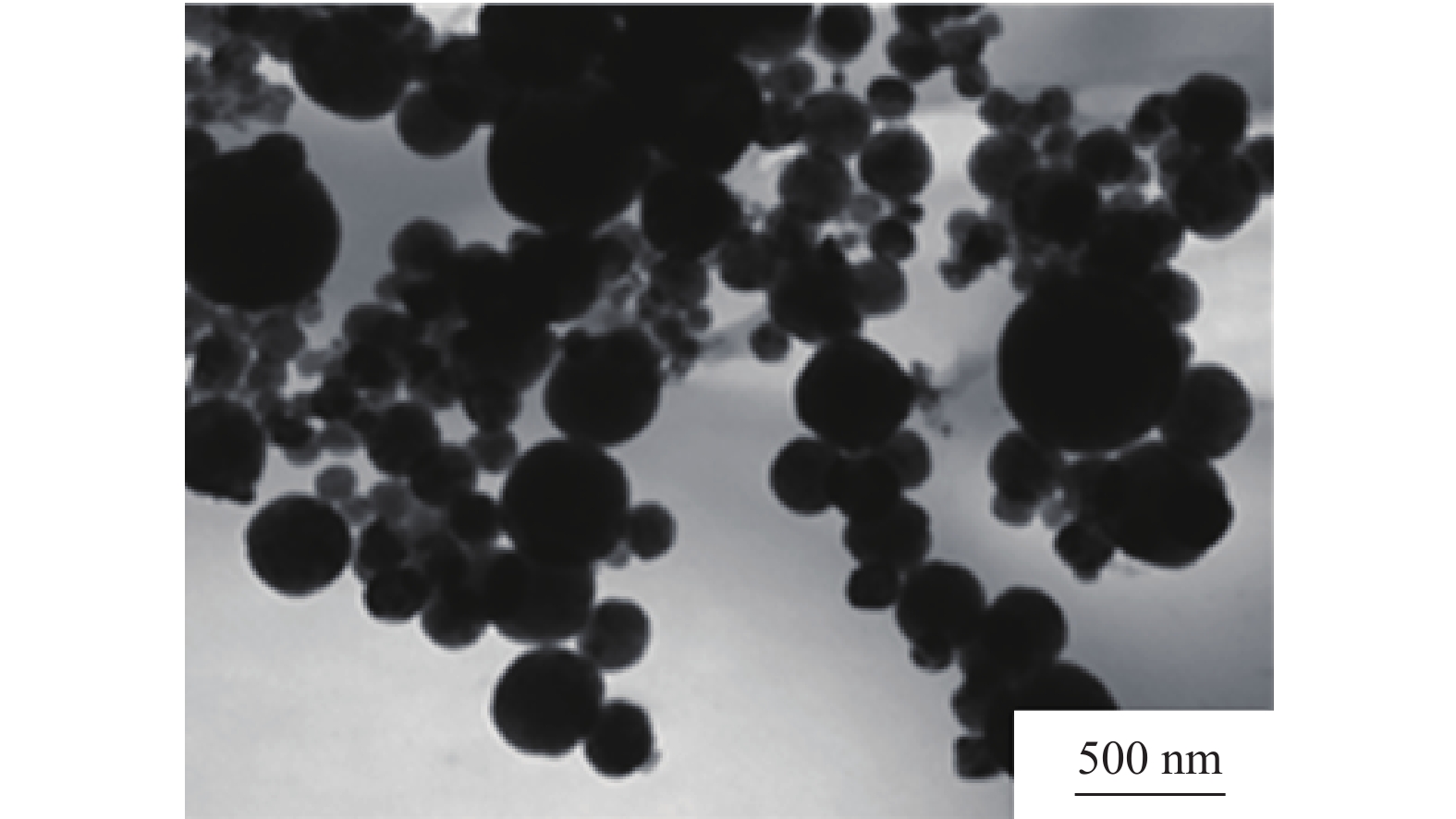
-
摘要: 通过两段氢气还原实验研究球形三氧化钼还原得到MoO2和Mo粉的显微形貌。结果表明:经一段还原后球形三氧化钼(β-MoO3)先变成α-MoO3,再生成立方形的γ-Mo4O11,最后形成α-MoO2;经二段还原得到Mo粉。MoO2形貌受还原温度和还原气氛影响较大,还原温度较低或者在还原气氛中引入水分时,MoO2为松散、细小的不规则形貌;还原温度较高或者还原气氛为大流量的干氢时,MoO2为薄片状,易板结。超细Mo粉的形貌主要受还原温度、水蒸汽分压和氢气分压的影响,还原温度低或者氢气流量较小,应尽量使水蒸汽分压和氢气分压的比值接近平衡常数,可得到大小均匀、分散的超细钼粉。Abstract: The morphology of MoO2 and Mo powders prepared by the two-stage hydrogen reduction experiment of the spherical molybdenum trioxide powders was studied. The results show that, the spherical molybdenum trioxide (β-MoO3) is transformed into α-MoO3 firstly, and then the square γ-Mo4O11 is regenerated to form α-MoO2 in first stage hydrogen reduction; Molybdenum powders are obtained in second stage hydrogen reduction. The morphology of MoO2 is greatly affected by the reduction temperature and reduction atmosphere, the MoO2 powders with fine and loose irregular morphology are obtained at low reduction temperature or by importing the water vapor into the reduction atmosphere; the MoO2 powders with flake and hardened are formed at the higher reduction temperature or in the dry hydrogen with large flow. The morphology of ultrafine Mo powders is mainly affected by the reduction temperature, the water vapor partial pressure, and the hydrogen partial pressure. When the reduction temperature is low or the hydrogen flow rate is small, the ratio of the water vapor partial pressure and the hydrogen partial pressure should be close to the equilibrium constant as far as possible, and the uniform and dispersed ultra-fine molybdenum powders can be obtained.
-
表 1 原料MoO3粉末化学成分(质量分数)
Table 1. Chemical composition of the raw MoO3 powders
% MoO3 Ca Al Si Fe S K C Cr ≥99.50 0.0015 0.0008 0.008 0.0048 0.0026 0.0029 0.0089 0.0011 -
[1] Xu K D. Material Science and Engineering of Molybdenum. Beijing: Metallurgical Industry Press, 2014徐克玷. 钼的材料科学与工程. 北京: 冶金工业出版社, 2014 [2] Cui Y Q, Zhou X W, He K. Research on the influence factors of the sublimation rate of molybdenum trioxide. China Molybdenum Ind, 2016, 40(1): 44崔玉清, 周新文, 何凯. 影响升华氧化钼升华率的因素研究. 中国钼业, 2016, 40(1): 44 [3] Zhao L P, Cai X N, Wang H Y, et al. Molybdenum trioxide: A new type negative electrode material for electric energy storage devices using Na+-based organic electrolytes. Chin J Appl Chem, 2017, 34(3): 262 doi: 10.11944/j.issn.1000-0518.2017.03.160239赵立平, 蔡兴楠, 王宏宇, 等. 三氧化钼—一种新型有机系钠离子储能器件负极材料. 应用化学, 2017, 34(3): 262 doi: 10.11944/j.issn.1000-0518.2017.03.160239 [4] Gao B, Zhang X J. Hydrothermal synthesis of single crystalα-MoO3 nanobelts and their formation mechanism. Mater Rev, 2017, 31(2): 112 doi: 10.11896/j.issn.1005-023X.2017.02.024高宾, 张晓军. 水热法合成MoO3单晶纳米带及其形成机理. 材料导报, 2017, 31(2): 112 doi: 10.11896/j.issn.1005-023X.2017.02.024 [5] Deng Y W. Controllable Preparation and Energy Storage Characteristics of Molybdenum Trioxide Nanoribbons [Dissertation]. Changsha: Hunan Normal University, 2020邓元文. 三氧化钼纳米带可控制备与储能特性研究[学位论文]. 长沙: 湖南师范大学, 2020 [6] Qi Y Y. Synthesis, Structure and Properties of One-Dimensional MoO3 Nanomaterials[Dissertation]. Wuhan: Wuhan University of Technology, 2007祁琰媛. 一维三氧化钼纳米材料的合成、结构与性能研究[学位论文]. 武汉: 武汉理工大学, 2007 [7] Wang G, Wang B X, Qu X H, et al. Progress in nano-sized molybdenum oxide preparation with different morphology. Chin J Rare Met, 2012, 36(6): 995 doi: 10.3969/j.issn.0258-7076.2012.06.025王戈, 王碧侠, 屈学化, 等. 不同形貌纳米三氧化钼制备研究进展. 稀有金属, 2012, 36(6): 995 doi: 10.3969/j.issn.0258-7076.2012.06.025 [8] Huang X F, Zou W, Ma C B. Property, use and production of β-type molybdenum trioxide. China Molybdenum Ind, 2000, 24(6): 29黄宪法, 邹炜, 马成兵. β型三氧化钼的性质、用途及生产. 中国钼业, 2000, 24(6): 29 [9] Zhou H, Zhou X W, Li G X, et al. Technological parameters study of MoO2 powder prepared by hydrogen reduction. Powder Metall Technol, 2015, 33(1): 3周华, 周新文, 李国新, 等. 氢气还原制备MoO2粉末工艺参数研究. 粉末冶金技术, 2015, 33(1): 3 [10] Wang L. Preparation of Ultrafine Molybdenum Oxides and the Kinetics Mechanism Studies Reduced by Gases[Dissertation]. Beijing: University of Science and Technology Beijing, 2018王璐. 超细氧化钼的制备及其气基还原动力学机理研究[学位论文]. 北京: 北京科技大学, 2018 [11] Liu Q P. Rate control of MoO3–MoO2 in the hydrogen reduction process. China Tungsten Ind, 2012, 27(5): 25刘秋萍. MoO3–MoO2氢还原过程中的速率控制. 中国钨业, 2012, 27(5): 25 [12] Liu Q P, Bai Y, Yi S F, et al. The effect of hydrogen dew point on the properties of molybdenum dioxide and molybdenum powder. China Tungsten Ind, 2018, 33(2): 42 doi: 10.3969/j.issn.1009-0622.2018.02.008刘秋萍, 白阳, 弋社峰, 等. 氢气露点对二氧化钼和钼粉性能的影响. 中国钨业, 2018, 33(2): 42 doi: 10.3969/j.issn.1009-0622.2018.02.008 [13] Zeng Y, Fu X J, Wang N, et al. Influence factors of oxygen content in small-particle molybdenum powder. Foundry Technol, 2015, 36(7): 1672曾毅, 付小俊, 王娜, 等. 小粒度钼粉氧含量的影响因素研究. 铸造技术, 2015, 36(7): 1672 [14] Li G Z, Wei X Y, Fu C W. Morphology evolution during production process of molybdenum powders. China Molybdenum Ind, 2017, 41(3): 52李光宗, 魏修宇, 傅崇伟. 钼粉制粉过程中的粉体形貌演变. 中国钼业, 2017, 41(3): 52 [15] Bolitschek J, Luidold S, O'Sullivan M. A study of the impact of reduction conditions on molybdenum morphology. Int J Refract Met Hard Mater, 2018, 71: 325 doi: 10.1016/j.ijrmhm.2017.11.037 [16] Du F J, Wang L, Yu Y. Hydrogen reduction processes of MoO2 to metal Mo with change of temperature. Chin J Rare Met, 2020, 44(11): 1201杜风娇, 王璐, 余岳. 温度对氢气还原二氧化钼制备钼粉的影响. 稀有金属, 2020, 44(11): 1201 -




 下载:
下载: