-
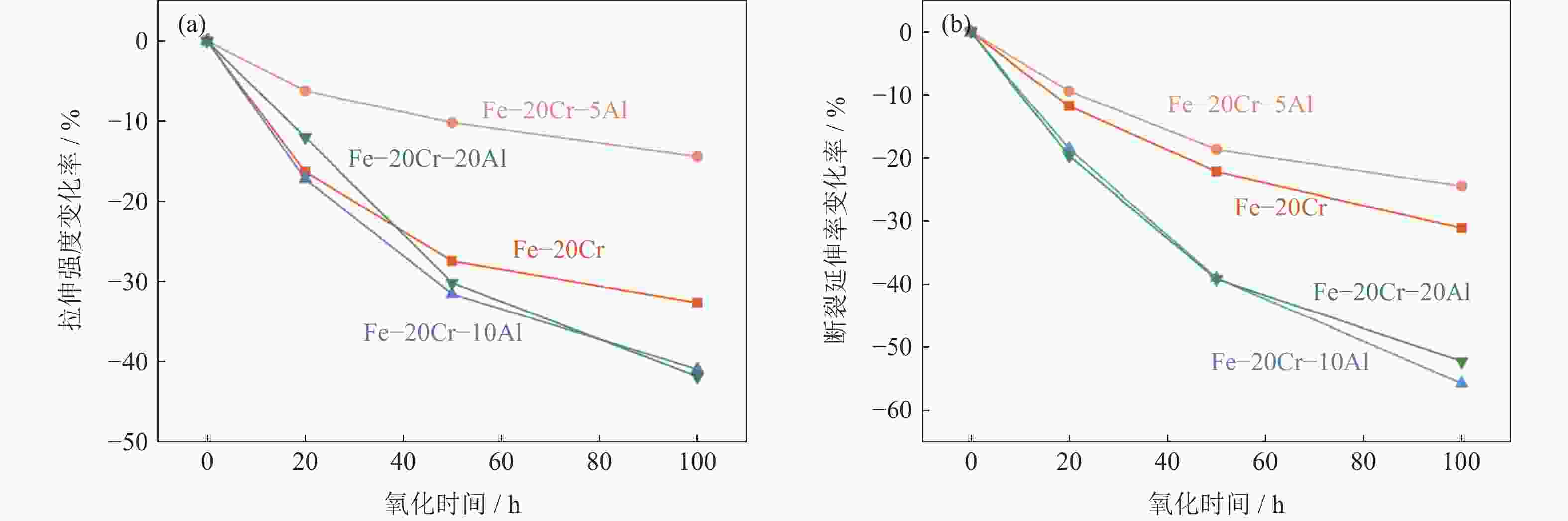
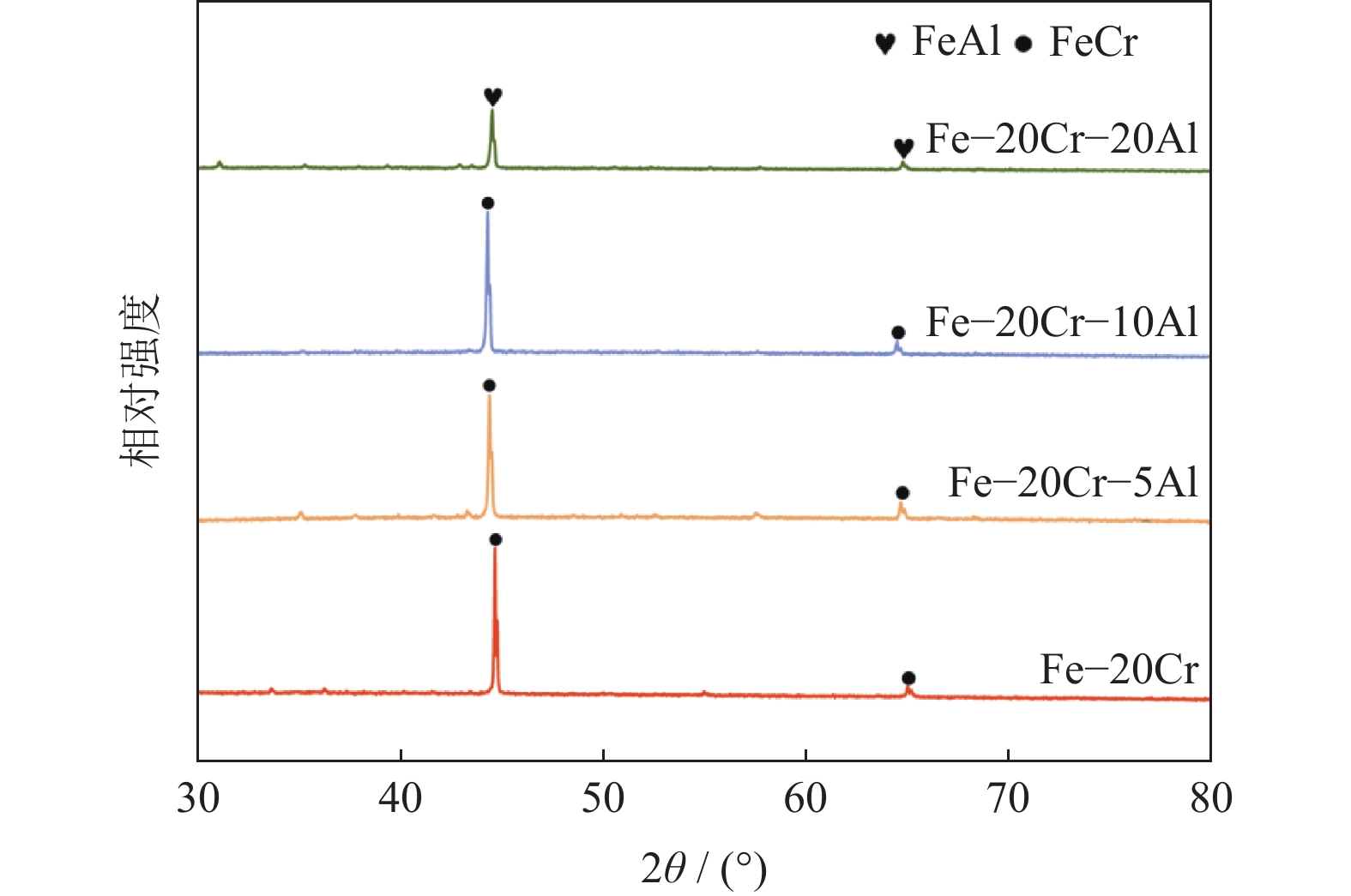
摘要: 铁铬铝(FeCrAl)合金是一种典型的耐热合金,由其开发而成的耐高温金属多孔材料已经在煤气净化、高温催化剂载体等方面获得了广泛的应用。在反应合成制备FeCrAl合金多孔材料过程中,铝质量分数在多孔材料孔结构、力学性能及抗氧化性能等方面具有重要影响。本文在Fe‒20%Cr合金(质量分数)基础上添加不同质量分数的铝粉(0~20%),以铁、铝、铬元素混合粉为原料,通过反应合成方法制备了一系列FeCrAl合金多孔材料(Fe‒20Cr‒xAl,x=0~20%,质量分数),研究了铝质量分数对Fe‒20Cr‒xAl多孔材料物相、孔结构、力学性能以及抗氧化性能的影响。结果表明,添加5%铝(质量分数)的Fe‒20Cr‒xAl多孔材料具有较优的孔隙度和力学性能,同时在600~800 ℃高温氧化实验中表现出最优的抗氧化性能和力学性能稳定性。Abstract: The typical heat-resistant FeCrAl alloys have recently been developed as the high-temperature resistant porous materials which are applied widely in the fields of coal-gas purification and high-temperature catalyst carrier. The aluminum mass fraction has an important influence on the pore structure, mechanical property, and oxidation resistance of the FeCrAl alloy porous materials prepared by the reactive synthesis. In this paper, a series of Fe‒20Cr‒xAl alloy porous materials (x=0~20%, mass fraction) were prepared by the reactive synthesis using the mixed Fe, Cr, and Al elemental powders as the raw materials. The effects of Al mass fraction on the phase composition, pore structure, mechanical property, and oxidation resistance of the FeCrAl porous materials were investigated. The results show that, the synthesized Fe‒20Cr‒5Al porous materials possess the superior pore structure and mechanical property, and show the optimal oxidation resistance and mechanical property stability in the high temperature oxidation experiment at 600~800 ℃.
-
Key words:
- porous materials /
- intermetallic /
- FeCrAl /
- mechanical properties /
- high temperature oxidation
-
表 1 Fe‒20Cr‒xAl合金多孔材料在初始和稳定阶段的氧化速率常数和拟合系数(R2)
Table 1. Oxidation rate constants and the fitting coefficients (R2) of the porous Fe‒20Cr‒xAl materials in the initial and subsequent stable oxidation stages
试样 抛物线速率常数 / (%·h‒1) R2 线性速率常数 / (%·h‒1) R2 Fe‒20Cr (600 ℃) 8.7×10−3 0.80 4.0×10−3 0.99 Fe‒20Cr‒5Al(600 ℃) 5.2×10−4 0.86 2.5×10−4 0.89 Fe‒20Cr‒10Al(600 ℃) 1.4×10−3 0.69 3.0×10−4 0.99 Fe‒20Cr‒20Al(600 ℃) 1.7×10−3 0.72 6.3×10−4 0.98 Fe‒20Cr (700 ℃) 5.3×10−2 0.79 1.4×10−2 0.98 Fe‒20Cr‒5Al(700 ℃) 5.0×10−3 0.95 1.4×10−3 0.88 Fe‒20Cr‒10Al(700 ℃) 5.2×10−3 0.95 1.8×10−3 0.92 Fe‒20Cr‒20Al(700 ℃) 9.0×10−3 0.98 4.4×10−3 0.98 Fe‒20Cr (800 ℃) 9.0×10−2 0.90 2.1×10−2 0.96 Fe‒20Cr‒5Al (800 ℃) 1.5×10−2 0.98 5.0×10−3 0.99 Fe‒20Cr‒10Al(800 ℃) 1.9×10−2 0.97 6.5×10−3 0.98 Fe‒20Cr‒20Al(800 ℃) 4.0×10−2 0.87 1.1×10−2 0.99 -
[1] Zhang H B, Wang L F, Ma J L, et al. Reactive synthesis of porous FeAlCr intermetallics with enhanced mechanical property and oxidation resistance by introducing yttrium borides. Mater Chem Phys, 2021, 273: 124929 doi: 10.1016/j.matchemphys.2021.124929 [2] Zhang H B, Gao H Y, Yu H, et al. The role of Cr in the reactive synthesis of porous FeAlCr intermetallic compounds. Mater Chem Phys, 2020, 249: 123013 doi: 10.1016/j.matchemphys.2020.123013 [3] Yin L, Jurewicz T B, Larsen M, et al. Uniform corrosion of FeCrAl cladding tubing for accident tolerant fuels in light water reactors. J Nucl Mater, 2021, 554: 153090 doi: 10.1016/j.jnucmat.2021.153090 [4] Tan P, Li A J, Chen J M, et al. Sulfidation resistance of porous FeCrAl alloy. Mater Sci Forum, 2018, 933: 226 doi: 10.4028/www.scientific.net/MSF.933.226 [5] Liu Y Y, Zheng Q Y. Effect of aluminum powders on the oxidation resistance of Al2O3‒MgO composites. Powder Metall Technol, 2019, 37(5): 367刘玉瑛, 郑清瑶. 铝粉对Al2O3–MgO复合材料抗氧化性的影响. 粉末冶金技术, 2019, 37(5): 367 [6] Jiang G, Xu D, Feng P, et al. Corrosion of FeCrAl alloys used as fuel cladding in nuclear reactors. J Alloys Compd, 2021, 869: 159235 doi: 10.1016/j.jallcom.2021.159235 [7] Gussev M N, Field K G, Yamamoto Y. Design, properties, and weldability of advanced oxidation-resistant FeCrAl alloys. Mater Des, 2017, 129: 227 doi: 10.1016/j.matdes.2017.05.009 [8] Wang F, Xi Z P, Tang H P, et al. Research progress of porous Fe‒Al alloy materials. Powder Metall Technol, 2010, 28(6): 463王峰, 奚正平, 汤慧萍, 等. Fe‒Al 合金多孔材料研究进展. 粉末冶金技术, 2010, 28(6): 463 [9] Zhang H B, Liu X L, Jiang Y, et al. Direct separation of arsenic and antimony oxides by high-temperature filtration with porous FeAl intermetallic. J Hazard Mater, 2017, 338: 364 doi: 10.1016/j.jhazmat.2017.05.049 [10] Pauletto G, Vaccari A, Groppi G, et al. FeCrAl as a catalyst support. Chem Rev, 2020, 120(15): 7516 doi: 10.1021/acs.chemrev.0c00149 [11] Uvarov V I, Kapustin R D, Kirillov A O, et al. Nanoporous high-temperature filters based on Ti–Al ceramic SHS materials. Ceram Int, 2020, 46(14): 23180 doi: 10.1016/j.ceramint.2020.06.098 [12] Zhang H B, Gao H Y, Liu X L, et al. Reactive synthesis and assessment of porous Fe‒20.5Al‒18Cr intermetallic material:A comparative study with porous FeCrAl material produced from prealloyed powders. Sep Purif Technol, 2019, 220: 152 [13] Zhang G Y, Chu R, Zhang H, et al. The oxidation mechanism of FeCrAl alloy added with rare earth Y from first-principle study. Adv Mater Res, 2013, 853: 192 doi: 10.4028/www.scientific.net/AMR.853.192 [14] Dai X F, Lin X D, Zhang J, et al. Research progress of α-α' phase separation of Fe‒Cr‒Al alloys for accident tolerant fuel cladding. Trans Mater Heat Treat, 2021, 42(7): 13戴学峰, 林晓冬, 张杰, 等. 事故容错燃料包壳Fe‒Cr‒Al合金α-α′相分离的研究进展. 材料热处理学报, 2021, 42(7): 13 [15] Gao H Y, He Y H, Zou J, et al. Pore structure control for porous FeAl intermetallics. Intermetallics, 2013, 32: 423 doi: 10.1016/j.intermet.2012.08.030 [16] Jia W Q, Liu X B, Xu C L, et al. Recent progress on powder metallurgy in preparation of FeCrAl alloy for nuclear fuel cladding. Powder Metall Technol, 2022, 40(1): 22贾文清, 刘向兵, 徐超亮, 等. 粉末冶金法制备核燃料包壳FeCrAl合金研究进展. 粉末冶金技术, 2022, 40(1): 22 [17] He L X, Liu C H, Lin J H, et al. Microstructure, oxidation and corrosion properties of FeCrAl coatings with low Al content prepared by magnetron sputtering for accident tolerant fuel cladding. J Nucl Mater, 2021, 551: 152966 doi: 10.1016/j.jnucmat.2021.152966 [18] Jiang Y, He Y H, Gao H Y. Recent progress in porous intermetallics: Synthesis mechanism, pore structure, and material properties. J Mater Sci Technol, 2021, 74: 89 doi: 10.1016/j.jmst.2020.10.007 [19] Yost A R, Erdeniz D, Puente A E P Y, et al. Effect of diffusion distance on evolution of Kirkendall pores in titanium-coated nickel wires. Intermetallics, 2019, 104: 124 doi: 10.1016/j.intermet.2018.10.020 [20] Gao H Y, He Y H, Zou J, et al. Tortuosity factor for porous FeAl intermetallics fabricated by reactive synthesis. Trans Nonferrous Met Soc China, 2012, 22(9): 2179 doi: 10.1016/S1003-6326(11)61446-5 [21] Butler T M, Weaver M L. Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J Alloys Compd, 2016, 674: 229 doi: 10.1016/j.jallcom.2016.02.257 [22] Düvel A, Romanova E, Sharifi M, et al. Mechanically induced phase transformation of γ-Al2O3 into α-Al2O3. Access to structurally disordered γ-Al2O3 with a controllable amount of pentacoordinated Al sites. J Phys Chem C, 2011, 115(46): 22770 [23] Kovarik L, Bowden M, Szanyi J. High temperature transition aluminas in δ-Al2O3/θ-Al2O3 stability range: Review. J Catal, 2021, 393: 357 doi: 10.1016/j.jcat.2020.10.009 [24] Tolpygo V K, Dryden J R, Clarke D R. Determination of the growth stress and strain in α-Al2O3 scales during the oxidation of Fe–22Cr–4.8Al–0.3Y alloy. Acta Mater, 1998, 46(3): 927 doi: 10.1016/S1359-6454(97)00306-6 [25] Li X F, Meng C Y, Xu X T, et al. Effect of Al content on high-temperature oxidation behavior and failure mechanism of CrAl-coated zircaloy. Corros Sci, 2021, 192: 109856 doi: 10.1016/j.corsci.2021.109856 -




 下载:
下载: