-
摘要: 以氮气为保护气氛,在820~980 ℃下用La2O3刻蚀人造金刚石单晶表面,研究稀土氧化物La2O3刻蚀对人造金刚石单晶性能的影响。利用扫描电子显微镜观测刻蚀后金刚石单晶不同晶面的表面形貌,通过人造金刚石单晶表面粗糙度、单颗粒抗压强度、抗冲击韧性和铜基结合剂金刚石节块抗弯强度来表征刻蚀前后金刚石单晶性能的变化。结果表明:La2O3对金刚石{100}面和{111}面的刻蚀是各向异性的;当刻蚀温度从820 ℃升高到980 ℃时,{100}面表面粗糙度从0.40 μm增加至2.28 μm,{111}面表面粗糙度从0.70 μm增加到3.32 μm,金刚石单颗粒的抗压强度由未刻蚀金刚石的576 N降低到最小530 N,冲击韧性由92.94%下降到89.21%。当金刚石体积分数为5%时,刻蚀后金刚石节块的抗弯强度增幅达到17.9%。Abstract: The surface of the synthetic diamond single crystal was etched by La2O3 at 820~980 ℃ with the nitrogen as the protective atmosphere to systematically study the effect of La2O3 etching on the properties of diamond single crystal. The surface morphologies of the etched diamond single crystal in the different crystal planes were observed by scanning electron microscope. The properties of the diamond single crystal before and after La2O3 etching were characterized by surface roughness, single particle compressive strength, impact toughness, and bending strength of Cu-based bonder diamond segments. The results show that, the La2O3 etching of the diamond in the {100} and {111} plane is anisotropic. When the etching temperature increases from 820 ℃ to 980 ℃, the surface roughness of {100} plane increases from 0.40 μm to 2.28 μm, while the surface roughness of {111} plane increases from 0.70 μm to 3.32 μm. The single particle compressive strength of the diamond decreases from 576 N (without etching) to the minimum of 530 N, and the impact toughness decreases from 92.94% to 89.21%. When the volume fraction of diamond is 5%, the bending strength of the etched diamond segment increases by 17.9%.
-
Key words:
- diamond single crystal /
- La2O3 /
- anisotropic /
- etching /
- surface roughness /
- mechanical properties
-
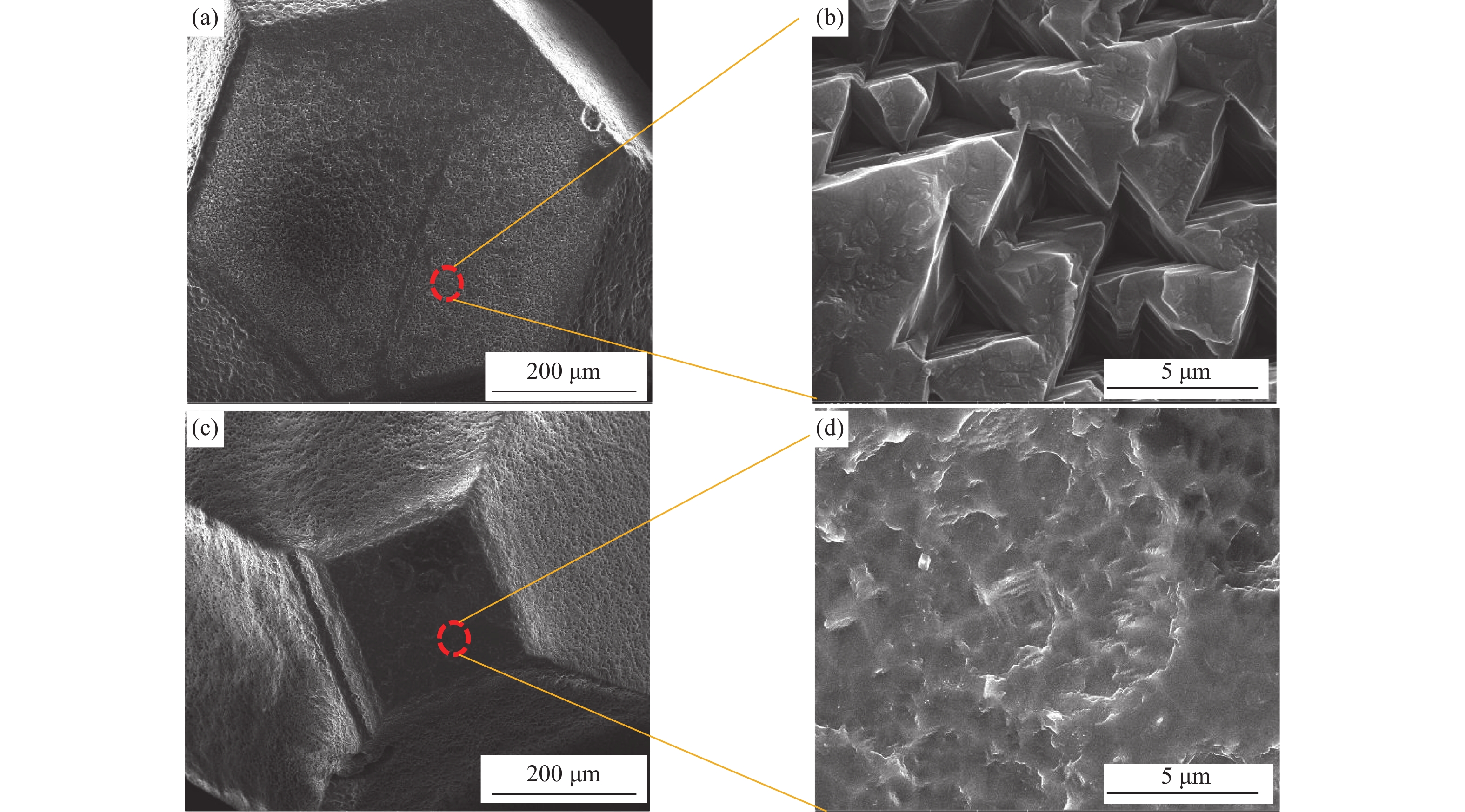
图 1 980 ℃刻蚀(La2O3)金刚石表面微观形貌:(a){111}面整体形貌;(b){111}面局部放大;(c){100}面整体形貌;(d){100}面局部放大
Figure 1. SEM images of the diamond surface etched by La2O3 at 980 ℃: (a) overall perspective of {111} plane; (b) local enlarged image of {111} plane; (c) overall perspective of {100} plane; (d) local enlarged image of {100}plane
表 1 不同温度下La2O3刻蚀后金刚石的抗冲击韧性
Table 1. Impact toughness of the diamond etched by La2O3 at different temperatures
温度 / ℃ 抗冲击韧性 / % 和原始金刚石的差值 / % 820 92.38 ‒0.56 860 91.42 ‒1.52 900 91.02 ‒1.92 940 90.22 ‒2.72 980 89.21 ‒3.73 -
[1] Chu C J, Pan C, Margrave J L, et al. F2, H2O, and O2 etching rates of diamond and the effects of F2, HF and H2O on the molecular O2 etching of (110) diamond. Diamond Relat Mater, 1995, 4(12): 1317 doi: 10.1016/0925-9635(95)00311-8 [2] Matumoto S, Setaka N. Thermal desorption spectra of hydrogenated and water treated diamond powders. Carbon, 1979, 17(6): 485 doi: 10.1016/0008-6223(79)90038-1 [3] de Theije F K, van der Laag N J, Plomp M, et al. A surface topographic investigation of {001} diamond surfaces etched in oxygen. Philos Mag A, 2000, 80(3): 725 doi: 10.1080/01418610008212078 [4] Smirnov W, Hees J J, Brink D, et al. Anisotropic etching of diamond by molten Ni particles. Appl Phys Lett, 2010, 97(7): 073117 doi: 10.1063/1.3480602 [5] Mehedi H, Arnault J, Eon D, et al. Etching mechanism of diamond by Ni nanoparticles for fabrication of nanopores. Carbon, 2013, 59: 448 doi: 10.1016/j.carbon.2013.03.038 [6] Wang J S, Wan L, Chen J, et al. Surface patterning of synthetic diamond crystallites using nickel powder. Diamond Relat Mater, 2016, 66: 206 doi: 10.1016/j.diamond.2016.04.010 [7] Nagai M, Nakanishi K, Takahashi H. Anisotropic diamond etching through thermochemical reaction between Ni and diamond in high temperature water vapour. Sci Rep, 2018, 8: 6687 doi: 10.1038/s41598-018-25193-2 [8] Konishi S, Ohashi T, Sugimoto W, et al. Effect of the crystal plane on the catalytic etching behavior of diamond crystallites by cobalt nanoparticles. Chem Lett, 2006, 35(11): 1216 doi: 10.1246/cl.2006.1216 [9] Wang J S, Wan L, Chen J, et al. Micropatterning of diamond crystallites via cobalt-catalyzed thermochemical etching. J Mater Sci, 2017, 52: 709 doi: 10.1007/s10853-016-0365-y [10] Mehedi H A, Hebert C, Ruffinatto S, et al. Formation of oriented nanostructures in diamond using metallic nanoparticles. Nanotechnology, 2012, 23: 455302 doi: 10.1088/0957-4484/23/45/455302 [11] Ohashi T, Sugimoto W, Takasu Y. Catalytic etching of synthetic diamond crystallites by iron. Appl Surf Sci, 2012, 258: 8128 doi: 10.1016/j.apsusc.2012.05.007 [12] Wang J S, Wan L, Chen J, et al. Anisotropy of synthetic diamond in catalytic etching using iron powder. Appl Surf Sci, 2015, 346: 388 doi: 10.1016/j.apsusc.2015.04.022 [13] Chepurov A, Sonin V, Shcheglov D, et al. A highly porous surface of synthetic monocrystalline diamond: Effect of etching by Fe nanoparticles in hydrogen atmosphere. Int J Refract Met Hard Mater, 2018, 76: 12 doi: 10.1016/j.ijrmhm.2018.05.011 [14] Shao Z Z, Su Y B, Zhang C S, et al. Iron etching of diamond in hydrogen-free atmosphere. Diamond Abras Eng, 2019, 39(5): 13 doi: 10.13394/j.cnki.jgszz.2019.5.0003邵增明, 苏彦宾, 张存升, 等. 无氢气氛下铁对金刚石的刻蚀. 金刚石与磨料磨具工程, 2019, 39(5): 13 doi: 10.13394/j.cnki.jgszz.2019.5.0003 [15] Xiao C J, Dou Z Q, Zhu Z D. Characterization and formation mechanism of surface morphology on diamond etched by Fe2O3 powder. Mater Rep, 2020, 34(7): 14045 doi: 10.11896/cldb.19070180肖长江, 窦志强, 朱振东. 氧化铁刻蚀金刚石表面形貌的表征及形成机理. 材料导报, 2020, 34(7): 14045 doi: 10.11896/cldb.19070180 [16] Xiao C J, Chen Y G, Li Z X. Study on etching of diamond monocrystal by MnO2. Diamond Abras Eng, 2019, 39(5): 1肖长江, 陈怡光, 栗正新. MnO2对金刚石单晶的刻蚀研究. 金刚石与磨料磨具工程, 2019, 39(5): 1 [17] Patel A R, Ramanathan S. Etch pits on diamond surfaces. Philos Mag, 1962, 7(80): 1305 doi: 10.1080/14786436208213164 [18] Patel A R. Mechanism of etching and development of etch figures on octahedral faces of diamond. Physica, 1962, 28: 44 doi: 10.1016/0031-8914(62)90090-3 [19] Li L Y, Che X, Zhang W, et al. Characterization and formation mechanism of pits on diamond {100} face etched by molten potassium nitrite. Int J Refract Met Hard Mater, 2018, 71: 129 doi: 10.1016/j.ijrmhm.2017.11.011 [20] Achard J, Silva F, Brinza O, et al. Identification of etch-pit crystallographic faces induced on diamond surface by H2/O2 etching plasma treatment. Phys Status Solidi, 2009, 206(9): 1949 doi: 10.1002/pssa.200982210 [21] Tsubouchi N, Mokuno Y, Shikata S. Characterizations of etch pits formed on single crystal diamond surface using oxygen/hydrogen plasma surface treatment. Diamond Relat Mater, 2016, 63: 43 doi: 10.1016/j.diamond.2015.08.012 [22] Kuroshima H, Makino T, Yamasaki S, et al. Mechanism of anisotropic etching on diamond (111) surfaces by a hydrogen plasma treatment. Appl Surf Sci, 2017, 422: 452 doi: 10.1016/j.apsusc.2017.06.005 [23] Jiang H, Liu F B, Yan H J, et al. Etching effects of hydrogen plasma treatment on diamond surfaces. Surf Coat Technol, 2019, 363: 12 doi: 10.1016/j.surfcoat.2019.02.007 [24] Wu H H, Zhang Z L, Sang L W, et al. Precise characterization of atomic-scale corrosion of single crystal diamond in H2 plasma based on MEMS/NEMS. Corros Sci, 2020, 170: 108651 doi: 10.1016/j.corsci.2020.108651 [25] Pan Y P, He X B, Ren S B, et al. Optimized thermal conductivity of diamond/Cu composite prepared with tungsten-copper-coated diamond particles by vacuum sintering technique. Vacuum, 2018, 153: 74 doi: 10.1016/j.vacuum.2018.03.052 [26] Wei C L, Xu X, Wei B Z, et al. Titanium coating on the surface of diamond particles by a novel rapid low-temperature salt bath plating method. Chem Phys Lett, 2020, 761: 15 [27] Morofushi Y, Matsushita H, Miki N. Microscale patterning of single crystal diamond by thermochemical reaction between sidero-metal and diamond. Precis Eng, 2011, 35(3): 490 doi: 10.1016/j.precisioneng.2011.03.003 [28] Wang J S. Study on Etching of Synthetic Diamond Crystallites by Iron Group Metals and Iron-Group Metal Salts [Dissertation]. Changsha: Hunan University, 2016王俊沙. 铁族金属及其盐对人造金刚石单晶腐蚀研究[学位论文]. 长沙: 湖南大学, 2016 [29] Xiao C J, Chen Y G, Li X L, et al. Method and mechanism analysis of improving the holding force between Ni-coated diamond and Cu-matrix bonding. Powder Metall Technol, 2020, 38(1): 25肖长江, 陈贻光, 栗晓龙, 等. 镀Ni金刚石与铜基结合剂间把持力的提高方法和机理分析. 粉末冶金技术, 2020, 38(1): 25 -




 下载:
下载: