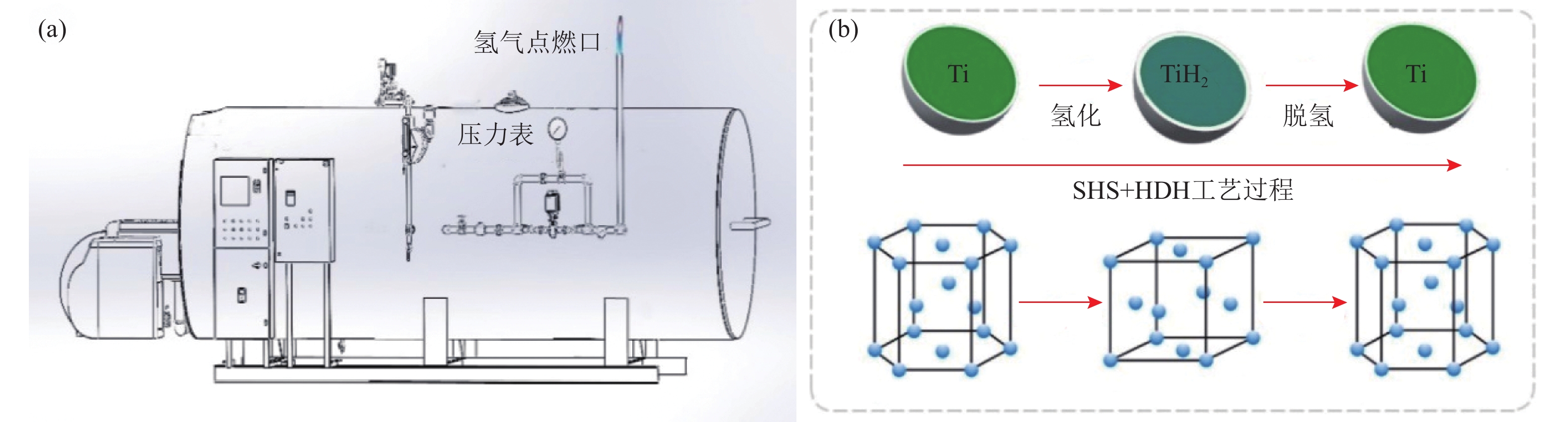
Optimization on hydrogenation-dehydrogenation preparation of titanium powders by SHS
-
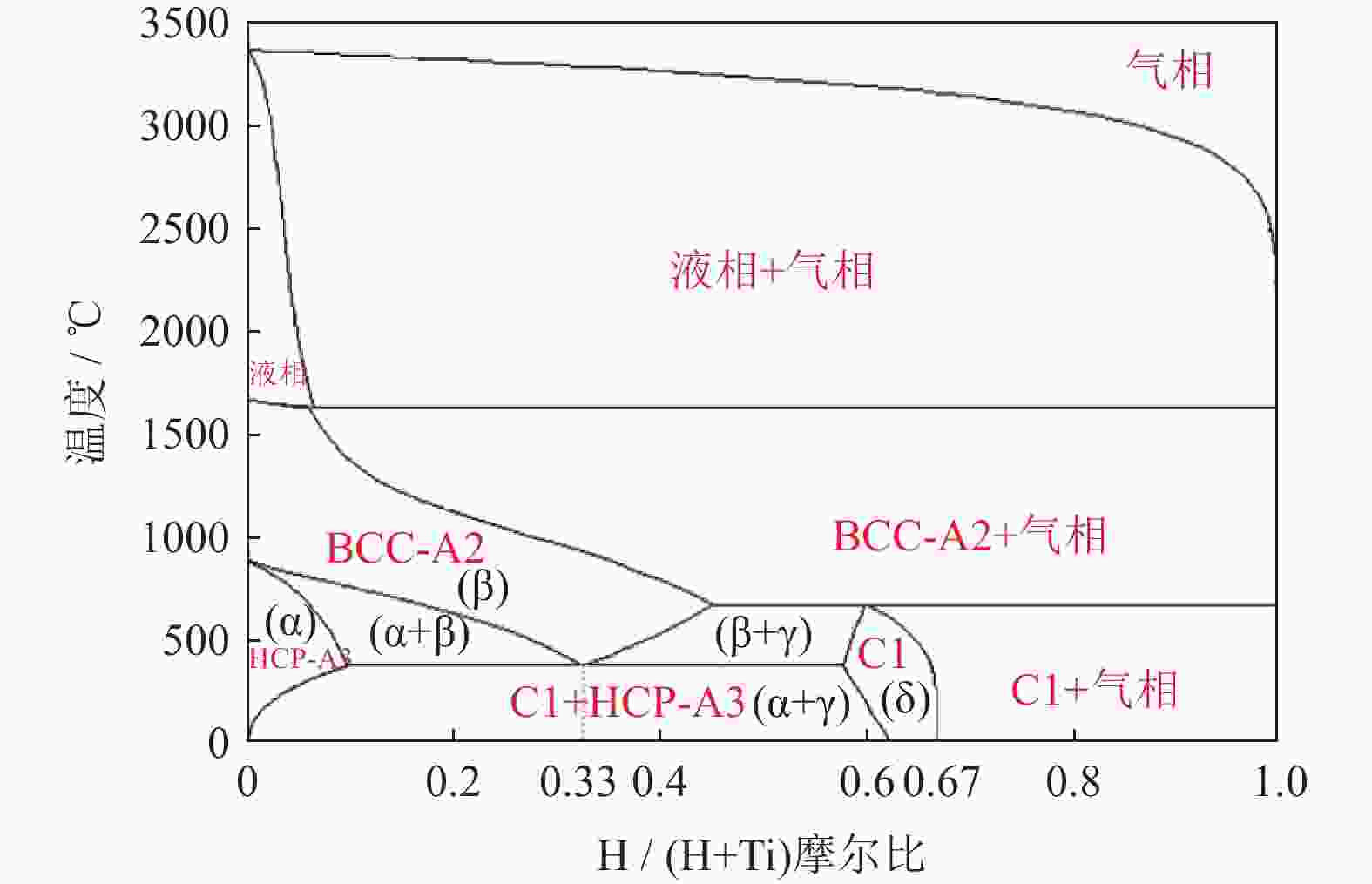
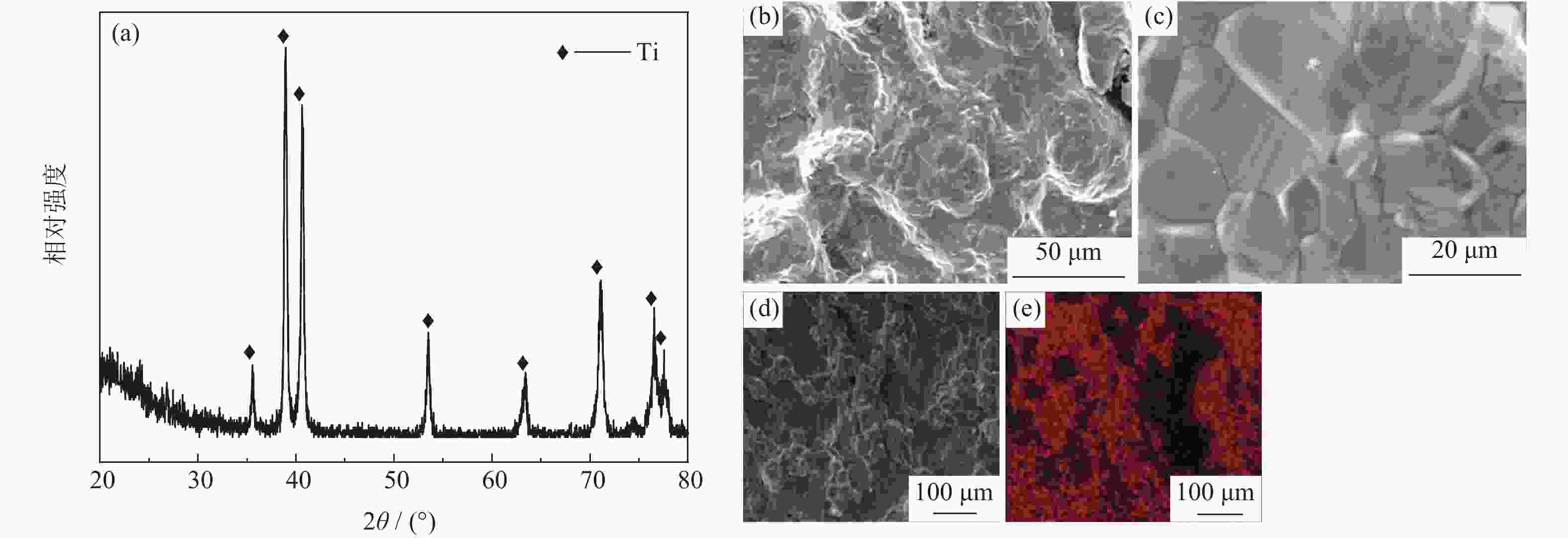
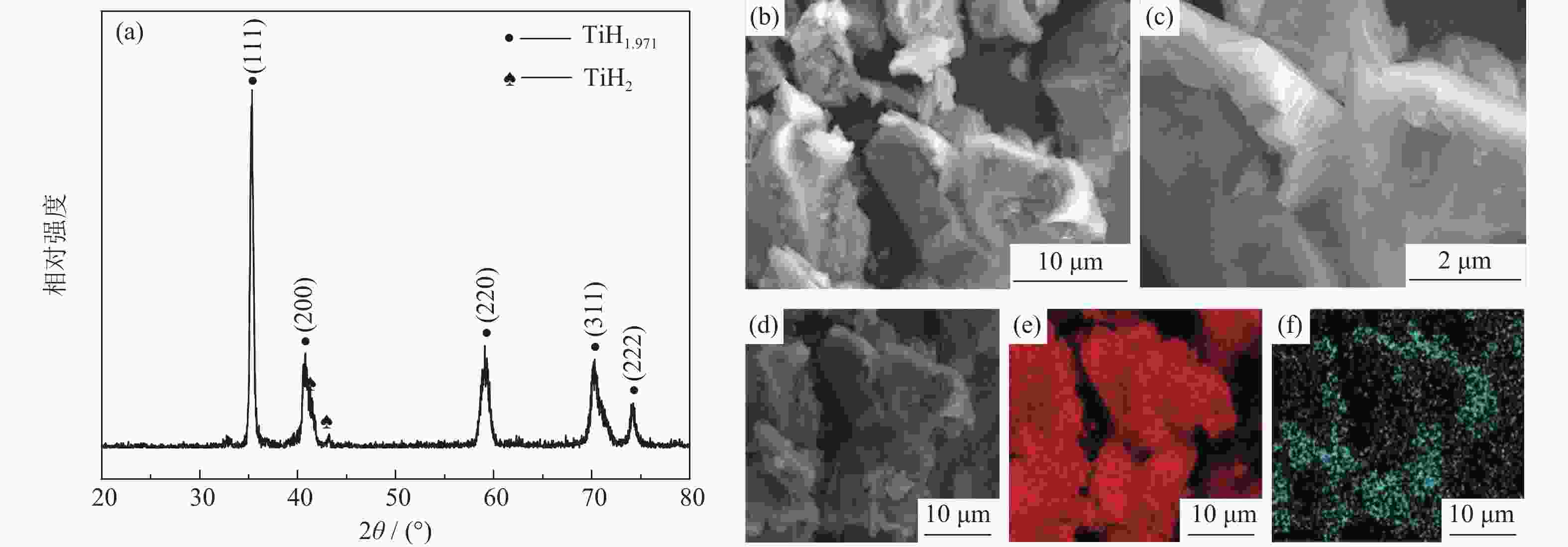
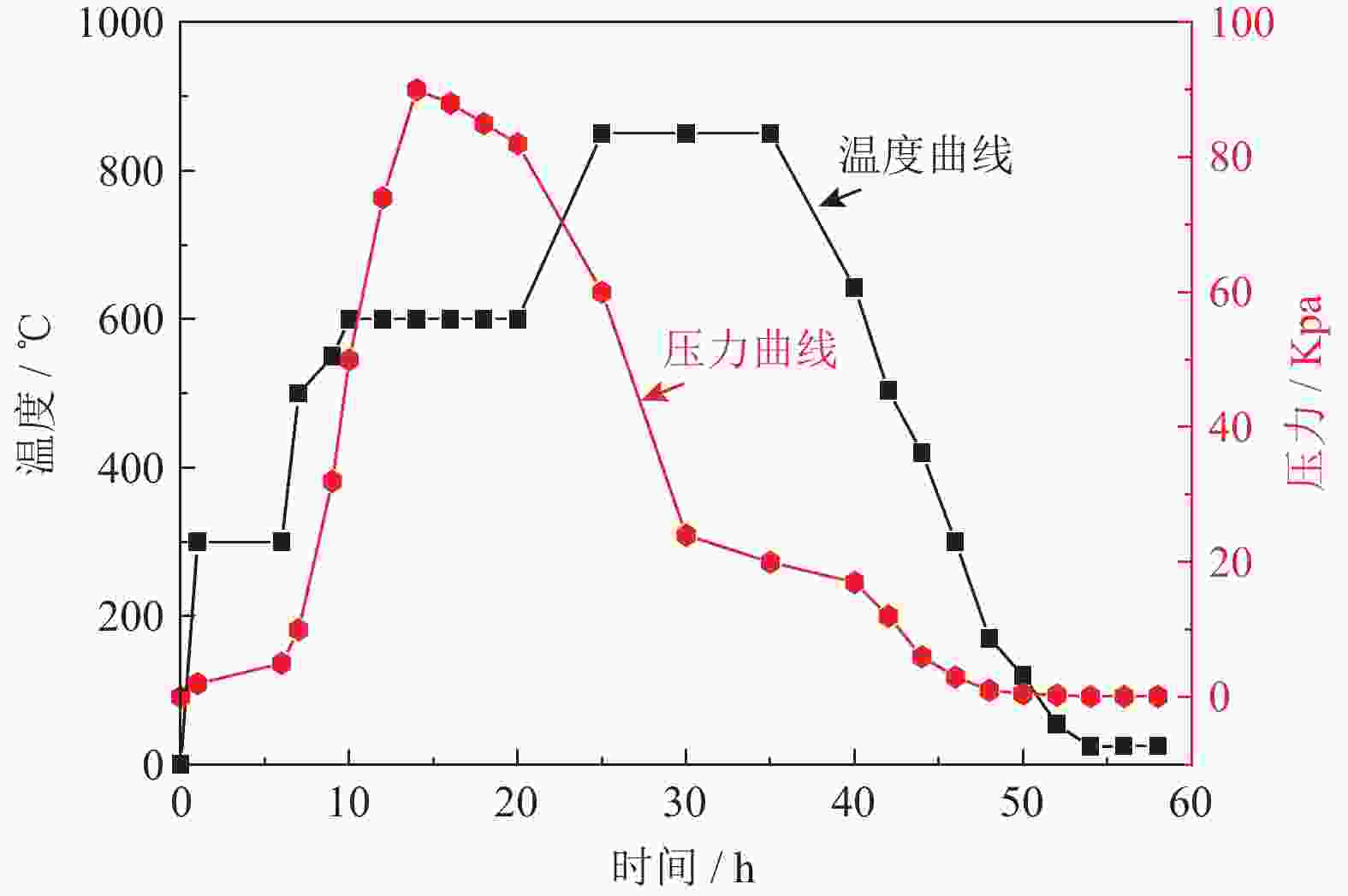
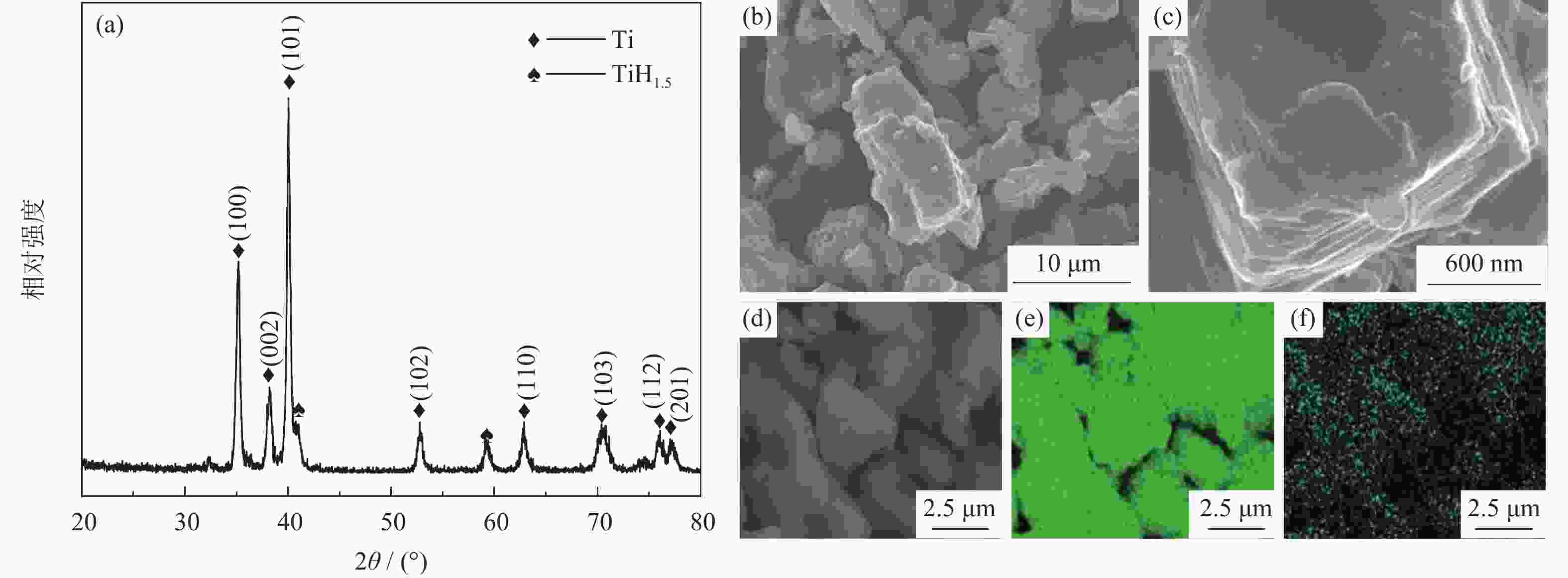
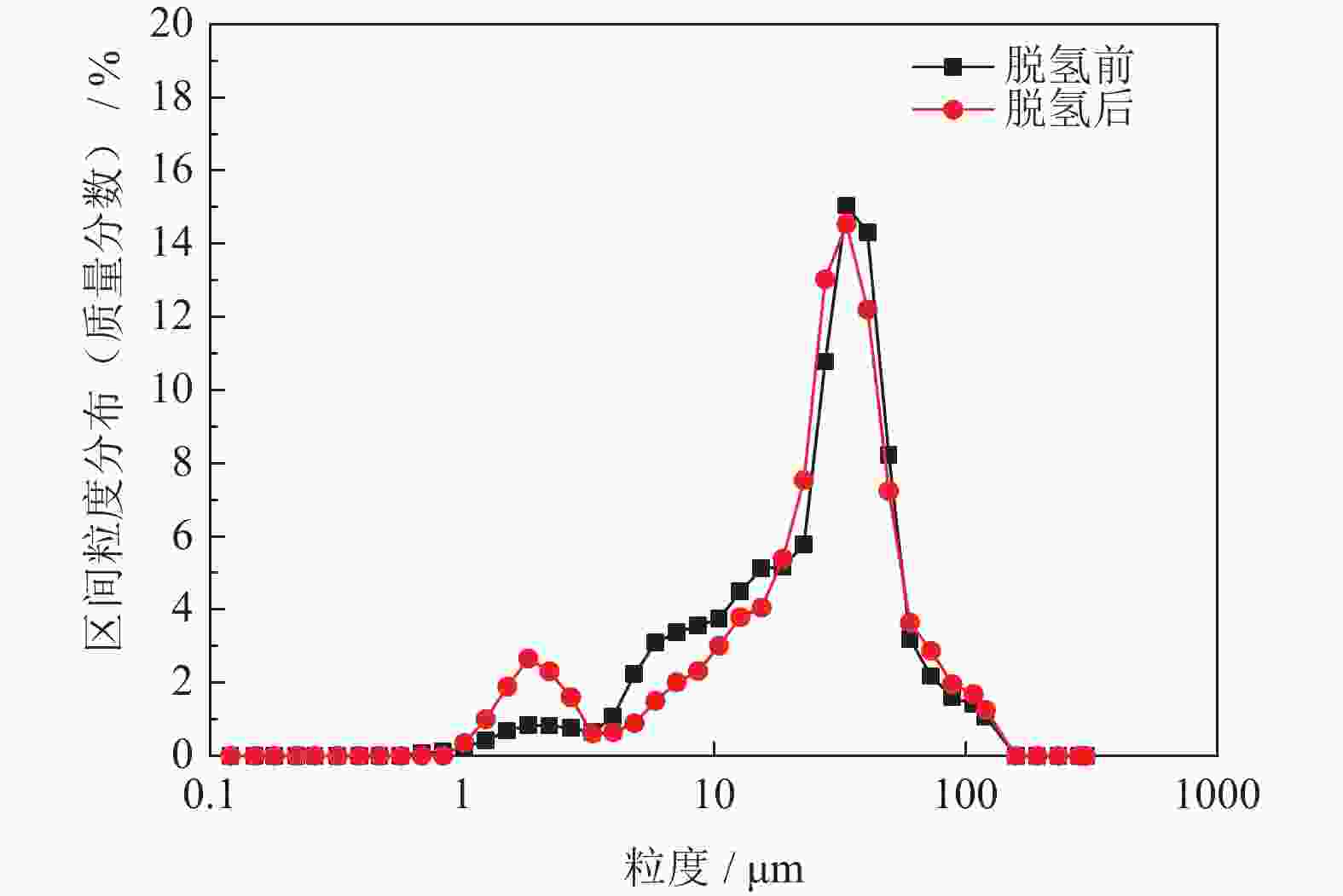
摘要: 为优化自蔓延高温合成法氢化脱氢制备钛粉工艺过程,改变传统钢球球磨为闭环气流研磨,改变传统抽空脱氢工艺为减压点燃工艺,研究了优化工艺下自蔓延高温合成法氢化和脱氢前后样品微观结构、物相组成、化学成分和粒度分布。结果表明,自蔓延高温合成氢化制得的氢化钛氢含量较高(4.662%,质量分数),闭环气流研磨氢化后样品粒度均匀,粒度分布范围为40~250 μm。与传统抽空脱氢工艺相比,减压点燃脱氢工艺有助于控制钛粉样品中N、O、C含量。Abstract: To optimize the hydrogenation-dehydrogenation process for preparing titanium powders by self-propagating high-temperature synthesis (SHS) method, the traditional steel ball milling process was replaced by the new closed-loop air current grinding process, and the traditional evacuation process for dehydrogenation process was replaced by the decompression-ignition process. The microstructure, phase component, chemical composition, and particle size distribution of the samples prepared by the optimization process were studied. In the results, the hydrogen mass fraction in the titanium hydride samples is high (4.662%) after SHS hydrogenation, and the particle size distribution of the TiH2 particles is uniform with the range of 40~250 μm after the closed-loop air flow grinding process. The new dehydrogenation process is beneficial to control the N, O, and C content in the titanium powder samples.
-
表 1 试样化学成分(质量分数)
Table 1. Chemical composition of samples
% 样品及状态 Si Fe Cl C N O Mn Mg H Sn Ni Cr Al Cu 海绵钛原料 0.005 0.017 0.040 0.006 0.003 0.051 0.001 0.005 0.001 0.004 0.001 0.002 0.003 0.005 氢化后氢化钛粉末 0.004 0.016 0.050 0.008 0.004 0.062 0.001 0.004 4.662 0.003 0.001 0.004 0.002 0.004 气流磨后氢化钛粉末 0.004 0.018 0.050 0.008 0.008 0.071 0.001 0.003 4.654 0.003 0.001 0.006 0.002 0.004 脱氢后钛粉 0.001 0.024 0.030 0.003 0.006 0.072 0.001 0.005 0.035 0.002 0.002 0.008 0.003 0.004 -
[1] Zhang C L, He X, Liu C, et al. Record high Tc element superconductivity achieved in titanium. Nat Commun, 2022, 13: 5411 doi: 10.1038/s41467-022-33077-3 [2] Zhang D Y, Qiu D, Gibson M A, et al. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature, 2019, 576: 91 doi: 10.1038/s41586-019-1783-1 [3] Yang Y C, Pan Y, Lu X, et al. Research progress on particle-reinforced titanium matrix composites prepared by powder metallurgy method. Powder Metall Technol, 2020, 38(2): 150杨宇承, 潘宇, 路新, 等. 粉末冶金法制备颗粒增强钛基复合材料的研究进展. 粉末冶金技术, 2020, 38(2): 150 [4] Fang Z Z, Froes F H, Zhang Y. Extractive Metallurgy of Titanium. Amsterdam: Elsevier, 2020 [5] Sun P, Fang Z Z, Xia Y, et al. A novel method for production of spherical Ti−6Al−4V powder for additive manufacturing. Powder Technol, 2016, 301: 331 doi: 10.1016/j.powtec.2016.06.022 [6] Goso X, Kale A. Production of titanium metal powder by the HDH process. J Southern Afr Inst Min Metall, 2011, 111(3): 203 [7] Kim Y I, Kim D K, Kim I, et al. Enhancing spreadability of hydrogenation-dehydrogenation titanium powder and novel method to characterize powder spreadability for powder bed fusion additive manufacturing. Materi Des, 2022, 226: 111247 [8] Huang G, Cao X H, Long X G. Physical and chemical properties of titanium-hydrogen system. Mater Rev, 2006, 20(10): 128 doi: 10.3321/j.issn:1005-023X.2006.10.032黄刚, 曹小华, 龙兴贵. 钛-氢体系的物理化学性质. 材料导报, 2006, 20(10): 128 doi: 10.3321/j.issn:1005-023X.2006.10.032 [9] McCracken C, Barbis D. Production of fine titanium powders via the hydride-dehydride (HDH) process. Powder Inject Mould Int, 2008, 2(2): 55 [10] Song Y L, Dou Z B, Zhang T A, et al. Research progress on the extractive metallurgy of titanium and its alloys. Miner Process Extr Metall Rev, 2022, 42: 535 [11] Sytschev A E, Kovalev D Y, Busurin S M, et al. Influence of synthesis conditions on the structure and phase formation during the SHS hydration of titanium. Russ J Non-Ferrous Met, 2015, 56(1): 86 doi: 10.3103/S1067821215010150 [12] Hayazi N F, Wang Y, Chan S L I. Unlocking the metastable phases and mechanisms in the dehydrogenation process of titanium hydride. Mater Charact, 2020, 161: 110128 doi: 10.1016/j.matchar.2020.110128 [13] Ma Q, Francis H F. Titanium Powder Metallurgy. Amsterdam: Elsevier, 2015 [14] Li X Y, Zhang L, Qin M L, et al. Effect of jet milling processing on microstructure and mechanical properties of the sintered tungsten powders. Powder Metall Technol, 2021, 39(3): 251李星宇, 章林, 秦明礼, 等. 气流磨处理对烧结钨粉微观组织和力学性能的影响. 粉末冶金技术, 2021, 39(3): 251 [15] Dai K L. Dehydrogening Character, Compactibility and Sintering Performance of TiH2 Powder [Dissertation]. Changsha: Hunan University, 2009戴坤良. TiH2粉体脱氢特性和压制与烧结行为研究[学位论文]. 长沙: 湖南大学, 2009 [16] Chen C Q, Li S X, Lu K. The defor mation behavioes of gamma hydrides in titanium under cyclic straining. Acta Mater, 2003, 51(4): 931 doi: 10.1016/S1359-6454(02)00495-0 [17] Rasooli A, Divandari M, Shahverdi H R, et al. Kinetics and mechanism of titanium hydride powder and aluminum melt reaction. Int J Miner Metall Mater, 2012, 19(2): 165 doi: 10.1007/s12613-012-0533-2 [18] Luo S Z, Yang B F, Long X G. Research progress on tritide target in neutron generator. At Energy Sci Technol, 2002(Z1): 290 doi: 10.7538/yzk.2002.36.z1.0290罗顺忠, 杨本福, 龙兴贵. 中子发生器用氚靶的研究进展. 原子能科学技术, 2002(Z1): 290 doi: 10.7538/yzk.2002.36.z1.0290 [19] Matijasevic L B, Banhart J, Fiechter S, et al. Modification of titanium hydride for improved aluminium foam manufacture. Acta Mater, 2006, 54(7): 1887 doi: 10.1016/j.actamat.2005.12.012 [20] Li G M, Gan L H, Chen L W, et al. The formation and decomposition of titanium hydride. Chin J Appl Chem, 1998, 15(1): 77 doi: 10.3724/j.issn.1000-0518.1998.1.77李光明, 甘礼华, 陈龙武, 等. 氢化钛的制备及其分解. 应用化学, 1998, 15(1): 77 doi: 10.3724/j.issn.1000-0518.1998.1.77 [21] Li D W, Li J R, Li Z C, et al. Effect of heating rate on thermolysis reaction kinetics of TiH2. J Mater Metall, 2009, 8(3): 175李大武, 李继荣, 李展超, 等. 升温速率对TiH2热分解反应动力学的影响. 材料与冶金学报, 2009, 8(3): 175 [22] Wang H T, Lefler M, Fang Z Z, et al. Titanium and titanium alloy via sintering of TiH2. Key Eng Mater, 2010, 436: 157 doi: 10.4028/www.scientific.net/KEM.436.157 [23] Savvakin D H, Humenyak M M, Matviichuk M V, et al. Role of hydrogen in the process of sintering of titanium powders. Mater Sci, 2012, 47: 651 doi: 10.1007/s11003-012-9440-y [24] Bhosle V, Baburaj E G, Miranova M, et al. Dehydrogenation of TiH2. Mater Sic Eng A, 2003, 365(1): 190 [25] Hong Y, Qu T, Shen H S, et al. Titanium production through hydrogenation and dehydrogenation process. Chin J Rare Met, 2007, 31(3): 311洪艳, 曲涛, 沈化森, 等. 氢化脱氢法制备钛粉工艺研究. 稀有金属, 2007, 31(3): 311 [26] Bhosle V, Baburaj E G, Miranova M, et al. Dehydrogenation of anocrystalline TiH2 and consequent consolidation to form dense Ti. Metall Mater Trans A, 2003, 34: 2793 doi: 10.1007/s11661-003-0180-3 [27] Huang L J, Yu B X, Gao S J. Kinetics of hydrogen absorption and desorption by titanium. Metall Funct Mater, 1998, 5(3): 124黄利军, 虞炳西, 高树浚. 钛吸氢和放氢动力学. 金属功能材料, 1998, 5(3): 124 [28] Chen T, Yang C, Liu Z, et al. Revealing dehydrogenation effect and resultant densification mechanism during pressureless sintering of TiH2 powder. J Alloys Compd, 2021, 873: 159792 doi: 10.1016/j.jallcom.2021.159792 [29] Kennedy A R, Lopez V H. The decomposition behavior of as-received and oxidized TiH2 foaming-agent powder. Mater Sci Eng A, 2003, 357(1): 258 [30] Stepura G, Rosenband V, Gany A. A model for the decomposition of titanium hydride and magnesium hydride. J Alloys Compd, 2012, 513: 159 doi: 10.1016/j.jallcom.2011.10.012 [31] Hayazi N F, Wang Y, Chan S L I. Unlocking the metastable phases and mechanisms in the dehydrogenation process of titanium hydride. Mater Charact, 2020, 161: 110128 doi: 10.1016/j.matchar.2020.110128 [32] Chen T, Yang C, Liu Z, et al. Revealing dehydrogenation effect and resultant densification mechanism during pressureless sintering of TiH2 powder. J Alloys Compd, 2021, 873: 159792 doi: 10.1016/j.jallcom.2021.159792 -



 下载:
下载: